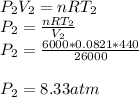Physics, 22.02.2020 02:49 nathanbrockdac
A sealed 26-m3 tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 440 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol·K = 0.0821 L·atm/mol·K. The final pressure of the gas is closest to
A) 0.31
B) 0.34
C) 0.33
D) 0.36
E) 0.29

Answers: 2


Another question on Physics

Physics, 21.06.2019 23:00
Pls pls pls pls ! describe, in your own words, how you found the epicenter of the earthquake.
Answers: 1

Physics, 22.06.2019 11:00
Which sound characteristic is not affected by the relative motion of an object
Answers: 2

Physics, 22.06.2019 14:40
Glass has a hardness that is in the middle of the hardness scale. what is the hardness of glass?
Answers: 1

You know the right answer?
A sealed 26-m3 tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K....
Questions



Geography, 27.03.2021 07:30


History, 27.03.2021 07:30



Mathematics, 27.03.2021 07:30


Mathematics, 27.03.2021 07:30

Mathematics, 27.03.2021 07:30

Chemistry, 27.03.2021 07:30




Physics, 27.03.2021 07:30

English, 27.03.2021 07:30



Mathematics, 27.03.2021 07:30