
Physics, 20.02.2020 08:16 madisontrosclair2
A cylinder contains oxygen gas at a pressure of 2.00 atm. The volume is 4.00 L, and the temperature is 300 K. Assume that the oxygen may be treated as an ideal gas. The oxygen is carried through the following processes:
(1) Heated at constant pressure from the initial state (state 1) to state 2, which has 450 K
(2) Cooled at constant volume to 250 K (state 3).
(3) Compressed at constant temperature to a volume of 4.00 L (state 4).
(4) Heated at constant volume to 300 K, which takes the system back to state 1.
A) Calculate Q and W for each of the four processes.
B) Calculate the net work done by the oxygen.
C) What is the effiency of this device as a heat engine? How does this effiiency compare with that of a Carnot-cycle engine operating between the same minimum temperatures of 250K and 450K?

Answers: 2


Another question on Physics

Physics, 21.06.2019 23:30
In which of the following cases is work being done on an object? question 2 options: pulling a trailer up a hill carrying a box down a corridor suspending a heavy weight with a strong chain pushing against a locked door
Answers: 2

Physics, 22.06.2019 06:30
Which features on mars point to the possibility of liquid water on the planet? impact craters with sharp rims volcanic cones with craters gullies and stream-like channels mountain ranges with faults
Answers: 1

Physics, 22.06.2019 07:10
Polly is pushing a box across the floor with a force of 30 n. the force of gravity is –8 n, and the normal force is 8 n. which value could describe the force of friction if polly could not move the box?
Answers: 2

You know the right answer?
A cylinder contains oxygen gas at a pressure of 2.00 atm. The volume is 4.00 L, and the temperature...
Questions




















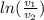 per unit mass of the gas
per unit mass of the gas

