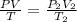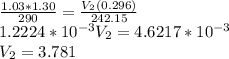A sealed balloon is filled with 1.30 L of helium at 17°C and 1.03 atm. The balloon rises to a point in the atmosphere where the pressure is 225 torr and the temperature is −31 °C. What is the change in the volume of the balloon as it ascends from 1.03 atm to a pressure of 225 torr?

Answers: 2


Another question on Physics

Physics, 22.06.2019 11:30
Water is siphoned from a large tank and discharges into the atmosphere through a 50-mm diameter tube. the end of the tube is b = 2.1 m below the tank bottom which is a = 7.4 m deep, and viscous effects are negligible. determine the maximum height h over which the water can be siphoned without cavitation occurring. atmospheric pressure is 101.4 kpa, and the water vapor pressure is 1.79 kpa (absolute)
Answers: 3

Physics, 22.06.2019 13:00
Nacidified solution was electrolyzed using copper electrodes. a constant current of 1.18 a caused the anode to lose 0.584 g after 1.52 ✕ 103 s. given that the charge of an electron is 1.6022 ✕ 10−19 c, calculate avogadro's number. assume that copper is oxidized to cu2+ ions.
Answers: 1

Physics, 22.06.2019 16:00
In which of the following is positive work done by a person on a suitcase
Answers: 1

Physics, 23.06.2019 01:00
Is the portion of the electromagnetic spectrum that has a frequency just above that of visible light.
Answers: 3
You know the right answer?
A sealed balloon is filled with 1.30 L of helium at 17°C and 1.03 atm. The balloon rises to a point...
Questions


Mathematics, 16.10.2020 18:01

Chemistry, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01




Physics, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01

Arts, 16.10.2020 18:01



Mathematics, 16.10.2020 18:01