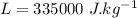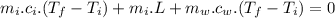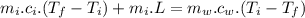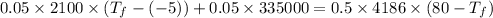Physics, 18.02.2020 02:22 cherylmorton7302
In an insulated container, 0.50 kg of water at 80°C is mixed with 0.050 kg of ice at −5.0°C. After a while, all the ice melts, leaving only the water. Find the final temperature of the water. The freezing point of water is 0°C.

Answers: 1


Another question on Physics

Physics, 21.06.2019 22:30
The law of conservation of energy states that energy cannot be created or , energy can be from one substance to another. the first blank is ' destroyed ' and for the second blank is ' transferred '
Answers: 1

Physics, 21.06.2019 23:00
Will you chech and finish these for me, because i am stumped with them.
Answers: 1

Physics, 22.06.2019 09:30
The graph represents the distance a car travels over time while on the highway. which statement about the car's trip is accurate? a) the car does not move over time. b) the car travels at a constant velocity. c) the car's velocity increases constantly over time. d) the car's velocity decreases constantly over time.
Answers: 1

Physics, 22.06.2019 11:30
1. a camcorder has a power rating of 19 watts. if the output voltage from its battery is 7 volts, what current does it use?answer units 2. a 1.5m wire carries a 6 a current when a potential difference of 57 v is applied. what is the resistance of the wire? yourunits 3. a clothes dryer uses about 2 amps of current from a 240 volt line. how much power does it use? yourunits 4.
Answers: 1
You know the right answer?
In an insulated container, 0.50 kg of water at 80°C is mixed with 0.050 kg of ice at −5.0°C. After a...
Questions



Mathematics, 09.10.2020 23:01

History, 09.10.2020 23:01



Mathematics, 09.10.2020 23:01

Mathematics, 09.10.2020 23:01

English, 09.10.2020 23:01


English, 09.10.2020 23:01


Health, 09.10.2020 23:01





Geography, 09.10.2020 23:01


Physics, 09.10.2020 23:01

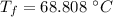
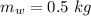 initial temperature of water,
initial temperature of water, 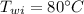 mass of ice,
mass of ice, 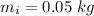 initial temperature of ice,
initial temperature of ice, 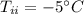 Specific heat capacity of water,
Specific heat capacity of water, 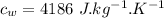 Specific heat capacity of ice,
Specific heat capacity of ice, 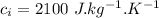 Latent heat of fusion of ice,
Latent heat of fusion of ice,