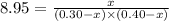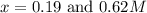A reaction A ( aq ) + B ( aq ) − ⇀ ↽ − C ( aq ) has a standard free‑energy change of − 5.43 kJ / mol at 25 °C. What are the concentrations of A , B , and C at equilibrium if, at the beginning of the reaction, their concentrations are 0.30 M, 0.40 M, and 0 M, respectively?

Answers: 2


Another question on Physics

Physics, 22.06.2019 07:00
We have a colorless transparent liquid. it looks like water. we seperated it into a solid and a liquid by evaporration and condention was this a chemichal or a physical seperation a. chemical seperation b. physical seperation
Answers: 3

Physics, 22.06.2019 16:50
Which best describes the first law of thermodynamics as compared to the second law of thermodynamics? a. the first law describes how thermal energy is conserved but not the direction it moves. b. the first law describes the direction thermal energy moves but not how it is conserved. c. the first law describes how thermal energy can be created but not how it can be destroyed. d. the first law describes how thermal energy can be destroyed but not how it can be created.
Answers: 1

Physics, 22.06.2019 20:00
Aturntable that spins at a constant 74.0 rpm takes 3.10 s to reach this angular speed after it is turned on. find its angular acceleration (in rad/s2), assuming it to be constant, and the number of degrees it turns through while speeding up.
Answers: 2

Physics, 23.06.2019 02:30
Aheavy boy and a lightweight girl are balanced on a massless seesaw. the boy moves backward, increasing his distance from the pivot point. what happens to the seesaw? it is impossible to predict without knowing additional information. the side the boy is sitting on will tilt downward. the side the girl is sitting on will tilt downward. nothing; the seesaw will remain balanced.
Answers: 2
You know the right answer?
A reaction A ( aq ) + B ( aq ) − ⇀ ↽ − C ( aq ) has a standard free‑energy change of − 5.43 kJ / mol...
Questions


Mathematics, 22.05.2020 05:03



Mathematics, 22.05.2020 05:03





Chemistry, 22.05.2020 05:03



Mathematics, 22.05.2020 05:03



Chemistry, 22.05.2020 05:03




Computers and Technology, 22.05.2020 05:03

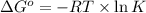
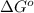 = standard Gibbs, free energy = -5.43 kJ/mol = -5430 J/mol
= standard Gibbs, free energy = -5.43 kJ/mol = -5430 J/mol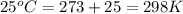
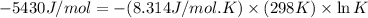
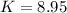
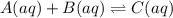
![K=\frac{[C]}{[A][B]}](/tpl/images/0509/5208/f1e20.png)