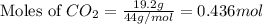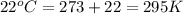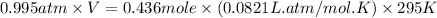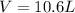Physics, 11.02.2020 23:31 kiarabermudez754
A 19.2g quantity of dry ice (solid carbon dioxide) is allowed to sublime (evaporate) in an apparatus. Calculate the expansion work done against a constant external pressure of 0.995 atm and at a constant temperature of 22 degrees C. Assume that the initial volume of dry ice is negligible and that CO2 behaves like an ideal gas.

Answers: 2


Another question on Physics

Physics, 23.06.2019 14:00
Multiply 5.036×102m by 0.078×10−1, taking into account significant figures.
Answers: 1

Physics, 23.06.2019 16:00
Give an example of a collision in real life. use the law of conservation of energy to describe the transfer of momentum. be sure and discuss the momentum before and after the collision occurs. you will need at least 3 sentences to thoroughly answer this question.
Answers: 1

Physics, 23.06.2019 17:30
Which is the most common use for infrared waves? to determine relative distance to provide heat to carry cell phone conversations to transmit signals
Answers: 1

Physics, 23.06.2019 18:30
Aforce of 6600 n is exerted on a piston that has an area of 0.010 [tex]m^{2}[/tex]. what area is rewired for a second piston to exert a force of 9900 n? use [tex]\frac{f_{1} }{a_{1} } = \frac{f_{2} }{a_{2} }[/tex]a. 0.0015 [tex]m^{2}[/tex]b. 66 [tex]m^{2}[/tex]c. 150 [tex]m^{2}[/tex]d. 0.006 [tex]m^{2}[/tex]
Answers: 1
You know the right answer?
A 19.2g quantity of dry ice (solid carbon dioxide) is allowed to sublime (evaporate) in an apparatus...
Questions

Mathematics, 04.11.2020 01:00

Mathematics, 04.11.2020 01:00

Mathematics, 04.11.2020 01:00



Mathematics, 04.11.2020 01:00

Advanced Placement (AP), 04.11.2020 01:00



Chemistry, 04.11.2020 01:00





Health, 04.11.2020 01:00


Mathematics, 04.11.2020 01:00



 .
.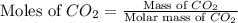
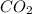 = 44 g/mole
= 44 g/mole