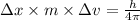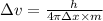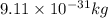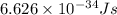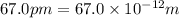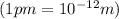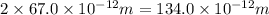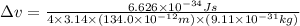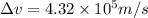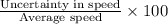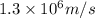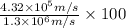An atom of carbon has a radius of 67.0 pm and the average orbital speed of the electrons in it is about 1.3 x 10⁶ m/s.
calculate the least possible uncertainty in a measurement of the speed of an electron in an atom of carbon. write your answer as a percentage of the average speed, and round it to significant digits.

Answers: 2


Another question on Physics

Physics, 22.06.2019 07:00
Photoelectrons with a maximum speed of 6.50 x 107 m/s are ejected from a surface in the presence of light with a frequency of 6.75 x 1014hz. if the mass of an electron is 9.10 x 10-31 kg, calculate in joules the maximum kinetic energy of a single electron. 3.84 x 10-15 j 1.92 x 10-15 j 5.92 x 10-23 j 3.07 x 10-16 j
Answers: 1

Physics, 22.06.2019 08:30
What properties of a moving object are used in determining the object's energy of motion
Answers: 2

Physics, 22.06.2019 10:30
An insulated 40 ft^3 rigid tank contains air at 50 psia and 120°f. a valve connected to the tank is now opened, and air is allowed to escape until the pressure inside drops to 25 psia. the air temperature during this process is kept constant by an electric resistance heater placed in the tank. determine the electrical work done during this process.
Answers: 2

Physics, 23.06.2019 00:00
Which is an advantage of subdividing science into different areas?
Answers: 3
You know the right answer?
An atom of carbon has a radius of 67.0 pm and the average orbital speed of the electrons in it is ab...
Questions







History, 02.08.2019 23:30



Health, 02.08.2019 23:30






Mathematics, 02.08.2019 23:30


English, 02.08.2019 23:30


History, 02.08.2019 23:30

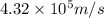
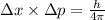 ...........(1)
...........(1)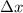 = uncertainty in position
= uncertainty in position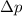 = uncertainty in momentum
= uncertainty in momentum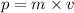
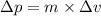 .......(2)
.......(2)