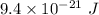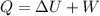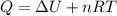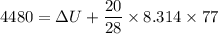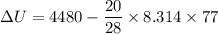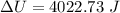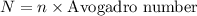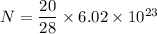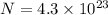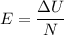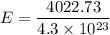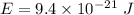Physics, 25.01.2020 04:31 Nessakona1
Suppose that we use a heater to boil liquid nitrogen (n2 molecules). 4480 j of heat turns 20 g of liquid nitrogen into gas. note that the latent heat is equal to the change in enthalpy, and that liquid nitrogen boils at 77 k. the system is kept at a constant pressure of 1 atm. 20) assuming that you can treat the gas as ideal gas and that the volume of the liquid compute the binding energy of a nitrogen molecule in the liquid. (the binding energy is the difference in internal energy per molecule between the liquid and gas) approximately zero,
a. 9.4 x 10-21 j
b. 3.8 х 1027 j
c. 4.2 x 10-18 j
d. 10-20 j e. 2.1 x 10-19 j

Answers: 2


Another question on Physics

Physics, 22.06.2019 00:40
Aballet student who learns with the of his instructor is demonstrating learning.
Answers: 3

Physics, 22.06.2019 07:20
Use the information presented in the graph to answer the questions. which segments show acceleration? which segment indicates that the object is slowing down? what is the velocity of segment b? what is the acceleration of segment b?
Answers: 3

Physics, 22.06.2019 11:00
Which of the following are guidelines to follow for obtaining accurate observations
Answers: 2

Physics, 22.06.2019 12:00
In which of the following would the particles move most rapidly? a. ice at -20 °c b. water at 20 °c c. steam at 110 °c d. boiling water e. ice at 0 °c
Answers: 1
You know the right answer?
Suppose that we use a heater to boil liquid nitrogen (n2 molecules). 4480 j of heat turns 20 g of li...
Questions


Mathematics, 26.04.2020 05:21

Biology, 26.04.2020 05:21


Mathematics, 26.04.2020 05:21



Mathematics, 26.04.2020 05:22

Physics, 26.04.2020 05:22

Chemistry, 26.04.2020 05:22

Mathematics, 26.04.2020 05:22

World Languages, 26.04.2020 05:24




Mathematics, 26.04.2020 05:38

English, 26.04.2020 05:39

Mathematics, 26.04.2020 05:39

Chemistry, 26.04.2020 05:39

Mathematics, 26.04.2020 05:39