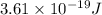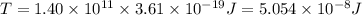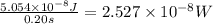Answers: 1


Another question on Physics

Physics, 21.06.2019 16:40
What is the internal energy of 4.00 mol of diatomic nitrogen gas n2 at 455k
Answers: 1

Physics, 22.06.2019 00:00
True or false: the revolution of the earth creates day and night as it rotates around the sun
Answers: 2

Physics, 22.06.2019 04:20
Awave is produced in a rope. the wave has a speed of 33 m/s and a frequency of 22 hz.
Answers: 3

Physics, 22.06.2019 12:00
In a set amount of time, a battery supplies 25j of energy to an electric circuit that includes two different loads. one of the loads produces 10 j of heat energy during this time interval. how much heat energy is produced by the second load in this time? explain your answer
Answers: 3
You know the right answer?
Calculate the minimum intensity of light in watts (j/s) required to eject 1.40 x 1011 electrons (eac...
Questions


Mathematics, 05.08.2021 15:30


Health, 05.08.2021 15:30




Mathematics, 05.08.2021 15:40

Biology, 05.08.2021 15:40

Social Studies, 05.08.2021 15:40


English, 05.08.2021 15:40




Law, 05.08.2021 15:40

Physics, 05.08.2021 15:40



Chemistry, 05.08.2021 15:40

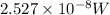 .
.