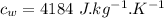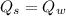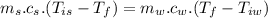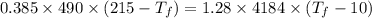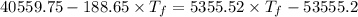Physics, 23.01.2020 00:31 simplydimps22owbohb
Heat transfer, specific heat. and calorimetrya 1.28-kg sample of water at 10.0 "c is in a calorimeter. you drop a piece of steel with a mass of 0.385 kg at 215 "c into it. after the sizzling subsides, what is the final equilibrium temperature? (make the reasonable assumptions that any steamproduced condenses into liquid water during the process of equilibration and that the evaporation and condensation don’taffect the outcome. as we’ll see in the next section.)

Answers: 1


Another question on Physics

Physics, 22.06.2019 03:40
Which types of electromagnetic waves have higher frequencies than the waves that make up ultraviolet light? check all that apply. radio waves infrared light microwaves gamma rays visible light x-rays
Answers: 2

Physics, 22.06.2019 08:00
You have a pick-up truck that weighed 4,000 pounds when it was new. you are modifying it to increase its ground clearance. when you are finished
Answers: 1

Physics, 22.06.2019 09:30
Which of these is not a possible type of energy transformation? a. electrical energy into light energy b. sound energy into nuclear energy c. potential energy into kinetic energy d. kinetic energy into mechanical energy
Answers: 1

Physics, 22.06.2019 12:50
The heliocentric and the geocentric models of the solar system included these central principles
Answers: 1
You know the right answer?
Heat transfer, specific heat. and calorimetrya 1.28-kg sample of water at 10.0 "c is in a calorimete...
Questions

English, 03.02.2021 23:50


History, 03.02.2021 23:50

Social Studies, 03.02.2021 23:50


Mathematics, 03.02.2021 23:50




Biology, 03.02.2021 23:50


Mathematics, 03.02.2021 23:50

History, 03.02.2021 23:50

Social Studies, 03.02.2021 23:50

History, 03.02.2021 23:50


Mathematics, 03.02.2021 23:50

Social Studies, 03.02.2021 23:50

Arts, 03.02.2021 23:50

Mathematics, 03.02.2021 23:50

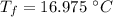
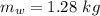 initial temperature of water,
initial temperature of water, 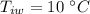 mass of steel,
mass of steel, 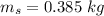 initial temperature of steel,
initial temperature of steel, 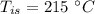 specific heat capacity of steel,
specific heat capacity of steel, 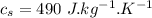 specific heat capacity of water,
specific heat capacity of water,