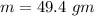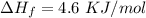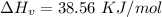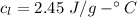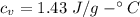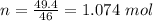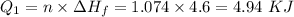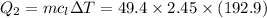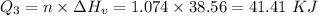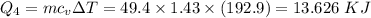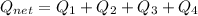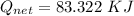Physics, 08.01.2020 02:31 bwright142
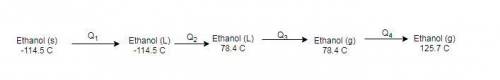
How much heat energy is required to convert 49.4 g of solid ethanol at − 114.5 ° c to gasesous ethanol at 140.7 ° c ? the molar heat of fusion of ethanol is 4.60 kj/mol , and its molar heat of vaporization is 38.56 kj/mol . ethanol has a normal melting point of − 114.5 ° c and a normal boiling point of 78.4 ° c . the specific heat capacity of liquid ethanol is 2.45 j / g ⋅ ° c , and that of gaseous ethanol is 1.43 j / g ⋅ ° c .

Answers: 3


Another question on Physics

Physics, 21.06.2019 22:30
The percent efficiency of a machine can never be 100% (or greater), because in the real world some energy is always converted into a. heat b. work c. input force d. output force
Answers: 1

Physics, 22.06.2019 05:30
Which of the following are considered noble gases? a. bromine b. neon c. argon d. chlorine
Answers: 1

Physics, 22.06.2019 07:20
Aman throws a football straight into the air. as it rises, it slows down. which type of energy is the football gaining?
Answers: 2

You know the right answer?
How much heat energy is required to convert 49.4 g of solid ethanol at − 114.5 ° c to gasesous ethan...
Questions


Social Studies, 12.03.2022 21:30


Social Studies, 12.03.2022 21:30


English, 12.03.2022 21:30

Spanish, 12.03.2022 21:30

Mathematics, 12.03.2022 21:30

English, 12.03.2022 21:30








Mathematics, 12.03.2022 21:40



English, 12.03.2022 21:50