
How much heat energy is required to convert 93.4 g of solid ethanol at − 114.5 ° c to gasesous ethanol at 149.8 ° c ? the molar heat of fusion of ethanol is 4.60 kj/mol , and its molar heat of vaporization is 38.56 kj/mol . ethanol has a normal melting point of − 114.5 ° c and a normal boiling point of 78.4 ° c . the specific heat capacity of liquid ethanol is 2.45 j / g ⋅ ° c , and that of gaseous ethanol is 1.43 j / g ⋅ ° c .

Answers: 3


Another question on Physics

Physics, 21.06.2019 17:00
Trumpeter a holds a b-flat note on the trumpet for a long time. person c is running towards the trumpeter at a constant velocity. person b is running away from the trumpeter at the same rate. person d is standing still the whole time. which person hears a frequency that is lower than the b-flat?
Answers: 1

Physics, 21.06.2019 23:00
We want to calculate the total metabolic heat generated by a singing canary taking into account heat transfer by radiation, convection and exhaling air. the air temperature is 20 oc, canary’s body internal and surface temperature is 33oc, external body surface convective heat transfer coefficient is 25.2 w/m2 .k, temperature difference between the inhaled and exhaled air is 4.3 oc, the ventilation rate is 0.74 cc of air per second, specific heat of air is 1.0066 kj/kg.k and density of air is 1.16 kg/m3 . assume the canary’s body to be a cylinder with 7 cm diameter and 9 cm length, and heat exchange is from the side as well as the top and bottom of cylinder. calculate 1) the net rate of heat lost by radiation, assuming heat gained by the bird through radiation from the surroundings is 11.5 w; 2) rate of heat transferred by convection to the surrounding air; 3) rate of heat transferred in the exhaling air without considering any internal evaporation; 4) total metabolic power.
Answers: 2

Physics, 22.06.2019 02:30
Awave travels at a speed of 5.2 m/s. if the distance between crests is 0.40 m, what is the frequency of the wave? use f=v/wavelength a. 13 hz b. 2.1 hz c. 0.077 hz d. 5.6 hz
Answers: 1

Physics, 22.06.2019 08:30
An automobile steering wheel is shown. what is the ideal mechanical advantage? if the ama is 8, what is the efficiency of the steering wheel?
Answers: 1
You know the right answer?
How much heat energy is required to convert 93.4 g of solid ethanol at − 114.5 ° c to gasesous ethan...
Questions


Mathematics, 04.12.2020 14:00

Mathematics, 04.12.2020 14:00

Social Studies, 04.12.2020 14:00


Chemistry, 04.12.2020 14:00


English, 04.12.2020 14:00


English, 04.12.2020 14:00

Mathematics, 04.12.2020 14:00

Mathematics, 04.12.2020 14:00

Biology, 04.12.2020 14:00

Mathematics, 04.12.2020 14:00

Mathematics, 04.12.2020 14:00


English, 04.12.2020 14:00


Physics, 04.12.2020 14:00

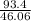 = 2.02 moles
= 2.02 moles

