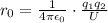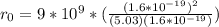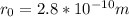The binding energy of a potassium chloride molecule (k0) is 4.43 ev. the ionization energy of a potassium atom is 4.3 ev, and the electron affinity of chlorine is 3.6 ev. use these data to estimate the equilibrium separation between the two atomsin the kci molecule. explain why your result is only an estimate and not a precise value.

Answers: 3


Another question on Physics

Physics, 22.06.2019 05:30
Mutt and jeff are running along a path each pushing a cart loaded with rocks. if mutt's wagon has a mass two times greater than jeff's and they want to stay together, arriving at their destination at the same time, what must happen? a) jeff should slow down so mutt can catch up. b) mutt must use half as much force to push his cart. c) mutt must use twice as much force to push his cart. d) mutt and jeff will use the same force, but mutt will take bigger steps.
Answers: 1

Physics, 22.06.2019 11:00
What is the rate of 12 liters of water moving through a water hose in 4.0 minutes?
Answers: 1

Physics, 22.06.2019 11:30
Achlorine atom has 17 protons and 18 neutrons. what is its mass number? what is its atomic number?
Answers: 2

Physics, 22.06.2019 20:00
Which is the most accurate name for the covalent compound p2o3?
Answers: 2
You know the right answer?
The binding energy of a potassium chloride molecule (k0) is 4.43 ev. the ionization energy of a pota...
Questions

Health, 02.01.2020 14:31

Mathematics, 02.01.2020 14:31

Mathematics, 02.01.2020 14:31



English, 02.01.2020 14:31

Social Studies, 02.01.2020 14:31



English, 02.01.2020 14:31

English, 02.01.2020 14:31


Mathematics, 02.01.2020 14:31

History, 02.01.2020 14:31

Social Studies, 02.01.2020 14:31

Mathematics, 02.01.2020 14:31




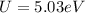
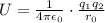
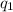 is the charge on one body,
is the charge on one body,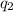 is the charge on the other body,
is the charge on the other body,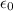 is the electric constant or permittivity of free space or permittivity of the vacuum
is the electric constant or permittivity of free space or permittivity of the vacuum