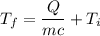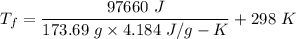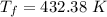Physics, 25.12.2019 01:31 rodderickjack
If 2.00 moles of lithium bromide are dissolved in 1000.0 grams of water at 25.0 °c, what is the final temperature of the water, assuming that all solutions have the same heat capacity as pure water (4.184 j/g-k)?

Answers: 1


Another question on Physics


Physics, 22.06.2019 11:30
Arocket starts from rest and moves upward from the surface of the earth. for the first 10.0 s of its motion, the vertical acceleration of the rocket is given by ay = 12.80 m/s32t, where the +y-direction is upward. (a) what is the height of the rocket above the surface of the earth at t = 10.0 s? b) what is the speed of the rocket when it is 325 m above the surface of the earth?
Answers: 3

Physics, 22.06.2019 16:00
Two balls, each with a mass of 0.5 kg, collide on a pool table. is the law of conservation of momentum satisfied in the collision? explain why or why not
Answers: 1

Physics, 22.06.2019 16:20
What is the mass of the water that is being heated? it requires 2,500 joules to raise a certain amount of water (c = 4.186 jig c) from 20.0°c to 60.0°c. o 159 o 40 g o 63 g o 80 g
Answers: 2
You know the right answer?
If 2.00 moles of lithium bromide are dissolved in 1000.0 grams of water at 25.0 °c, what is the fina...
Questions


English, 15.01.2020 14:31

History, 15.01.2020 14:31

History, 15.01.2020 14:31

Mathematics, 15.01.2020 14:31

History, 15.01.2020 14:31

Mathematics, 15.01.2020 14:31


French, 15.01.2020 14:31

English, 15.01.2020 14:31



Advanced Placement (AP), 15.01.2020 14:31


History, 15.01.2020 14:31

Physics, 15.01.2020 14:31


Mathematics, 15.01.2020 14:31


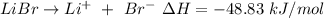
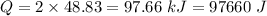
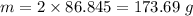
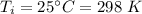
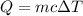
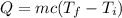
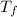 is the final temperature
is the final temperature