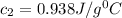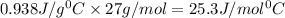Physics, 24.12.2019 21:31 jamiezanfardino1464
A23.5 g piece of aluminum metal is initially at 100.0°c. it is dropped into a coffee cup-calorimeter containing 130.0 g of water at a temperature of 23.0°c. after stirring, the final temperature of both copper and water is 26.0°c. assuming no heat losses, and that the specific heat capacity of water is 4.184 j/(g·°c), what is the molar heat capacity of aluminum, cm(al)?

Answers: 3


Another question on Physics


Physics, 22.06.2019 10:30
You are given two vectors a⃗ =−3.00ι^ 5.00j^ and b⃗ =5.00ι^ 2.00j^. let the counterclockwise angles be positive.
Answers: 3

Physics, 22.06.2019 17:30
Four objects each with charge +2.0×10−7c are located at the corners of a square whose sides are 2.0 m long. part a what quantities can be determined using this information? check all that apply. the electric force on a charged object placed at the center of the square. the mass of each object. the total electric potential energy of the system consisting of the four charged objects. part b find the electric force on a charged object placed at the center of the square.
Answers: 1

You know the right answer?
A23.5 g piece of aluminum metal is initially at 100.0°c. it is dropped into a coffee cup-calorimeter...
Questions


Mathematics, 08.01.2020 02:31

Physics, 08.01.2020 02:31


History, 08.01.2020 02:31

History, 08.01.2020 02:31

Mathematics, 08.01.2020 02:31

Biology, 08.01.2020 02:31


History, 08.01.2020 02:31

Biology, 08.01.2020 02:31

Biology, 08.01.2020 02:31


Computers and Technology, 08.01.2020 02:31

Biology, 08.01.2020 02:31


History, 08.01.2020 02:31



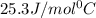
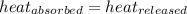
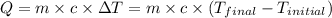
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0432/0719/09236.png) .................(1)
.................(1)
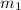 = mass of water = 130.0 g
= mass of water = 130.0 g
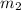 = mass of aluminiunm = 23.5 g
= mass of aluminiunm = 23.5 g
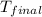 = final temperature
=
= final temperature
= 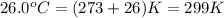
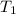 = temperature of water =
= temperature of water = 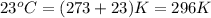
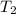 = temperature of aluminium =
= temperature of aluminium = 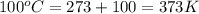
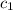 = specific heat of water=
= specific heat of water= 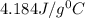
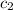 = specific heat of aluminium= ?
= specific heat of aluminium= ?![130.0\times 4.184\times (299-296)=-[23.5\times c_2\times (299-373)]](/tpl/images/0432/0719/5591a.png)