Physics, 24.12.2019 01:31 christylivingsowzxa2
For 0.37 moles of oxygen (02) gas at room temperature with active translational and rotational degrees of freedom.
1) how much heat must be added to in order to raise its temperature by 15 k with the pressure kept constant?
a. 100.4 j
b. 161.5 j
c. 69.2j
d. 19.4j
e. 935.3 j

Answers: 1


Another question on Physics

Physics, 22.06.2019 07:30
The slope of a velocity time graph over any interval of time gives the during that interval
Answers: 2

Physics, 22.06.2019 12:10
Consider a one meter long horizontal pipe with a constant 100 cm^2 cross sectional area. water flows rightward into the pipe at x = 0 with flow velocity 02m/sec at every point within the pipe intake area. at x=1, the rightward flow rate is 0.192 m/sec. assume the water is a conserved quantity in the pipe, so there must be a leak (a sink) somewhere in the pipe. 1. compute net volumetric flow of the source if the system to be in equilibrium. 2. now assume the pipe in the problem has no leaks. compute the net volumetric rate of change for the system.
Answers: 3

Physics, 22.06.2019 15:00
10 points! will mark brainiest! in a heat engine if 1,000 j of heat enters the system and the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2,000 j 1: write the equation2: list out your known variables 3: plug the numbers into the equations 4: solve 5: write your solution statement that includes initial energy and final energy added you so much!
Answers: 2

You know the right answer?
For 0.37 moles of oxygen (02) gas at room temperature with active translational and rotational degre...
Questions

English, 18.10.2019 11:30


Mathematics, 18.10.2019 11:30


History, 18.10.2019 11:30

Social Studies, 18.10.2019 11:30

Mathematics, 18.10.2019 11:30


Chemistry, 18.10.2019 11:30


Mathematics, 18.10.2019 11:30



Mathematics, 18.10.2019 11:30


Mathematics, 18.10.2019 11:30


Mathematics, 18.10.2019 11:30


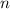 = Number of moles = 0.37
= Number of moles = 0.37  = Rise in the temperature of the oxygen gas = 15 K
= Rise in the temperature of the oxygen gas = 15 K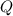 = heat added in order to raise the temperature
= heat added in order to raise the temperature 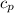 = specific heat at constant pressure = 3.5
= specific heat at constant pressure = 3.5