
Physics, 19.12.2019 06:31 kaywendel2008
The energy e of the electron in a hydrogen atom can be calculated from the bohr formula: =e−ryn2 in this equation ry stands for the rydberg energy, and n stands for the principal quantum number of the orbital that holds the electron. (you can find the value of the rydberg energy using the data button on the aleks toolbar.) calculate the wavelength of the line in the emission line spectrum of hydrogen caused by the transition of the electron from an orbital with =n10 to an orbital with =n8. round your answer to 3 significant digits.

Answers: 3


Another question on Physics

Physics, 21.06.2019 14:30
Does any one know what real love is and would anyone be willing to share it with me?
Answers: 2

Physics, 22.06.2019 09:30
For this car, predict the shape of a graph that shows distance (x) versus time (t). note that time is the independent variable and will be on the bottom axis. need asap
Answers: 1

Physics, 22.06.2019 12:30
Hydrogen atoms are excited by a laser to the =4n=4 state and then allowed to emit. what is the maximum number of distinct emission spectral lines (lines of different wavelengths) that can be observed from this system? 8 6 2 7 4 5 1 3 calculate the wavelength of the 4⟶14⟶1 transition. =λ=
Answers: 2

Physics, 22.06.2019 16:00
The electric field direction is defined by the direction of the force felt by (select one of the following answers): 1. a negative charge. 2. a positive charge. 3. both positive and negative charges.
Answers: 2
You know the right answer?
The energy e of the electron in a hydrogen atom can be calculated from the bohr formula: =e−ryn2 in...
Questions




History, 20.11.2019 06:31



History, 20.11.2019 06:31


Mathematics, 20.11.2019 06:31


Spanish, 20.11.2019 06:31

Chemistry, 20.11.2019 06:31

English, 20.11.2019 06:31


Chemistry, 20.11.2019 06:31

History, 20.11.2019 06:31


History, 20.11.2019 06:31

Mathematics, 20.11.2019 06:31

Biology, 20.11.2019 06:31

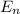 = - k²e² / 2m (1 / n²)
= - k²e² / 2m (1 / n²)
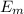 = - k²e² / 2m (1 /
= - k²e² / 2m (1 / 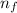 ² -1 /
² -1 / 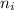 ²)
²)


