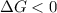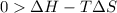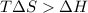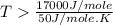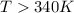One might be tempted to say that exothermic processes are always spontaneous since the system is emitting energy (heat) in order to reach a (preferred) lower energy state. however, as we have just investigated, the spontaneous process for polymers is endothermic. this reveals that we must consider entropy changes when determining the nature of spontaneity. the most probable configuration of a system and its surroundings, naturally, is the one that will be observed. the condition for spontaneity can be recast using the concept of the free energy of the system, where a change in free energy results both from changes in the enthalpy (which includes internal potential and kinetic energies) and the entropy (the number of states accessible to the system). δ g = δ h − t δ s.
an unknown chemical reaction undergoes an enthalpy change of δ h =17 kj/mol while the entropy increases by δ s =50 j/(mol * k).
above what temperature (in kelvin) does this reaction occur spontaneously?

Answers: 1


Another question on Physics

Physics, 21.06.2019 16:10
Zirconium has an hcp crystal structure and a density of 6.51 g/cm3. (a) what is the volume of its unit cell in m3? (b) if the c/a ratio is 1.593, compute the values of c and a.
Answers: 3

Physics, 22.06.2019 00:30
Next a skier is pulled by a tow rope up a frictionless ski slope that makes an angle of 15 with the horizontal. the rope moves parallel to the slope with a constant speed of 0.69 m/s. the force of the rope does 800 3 of work on the skier as the skier moves a distance of 8.4 m up the incline. (a) if the rope moved with a constant speed of 2.2 m/s how much work would the force of the rope do on the skier as the skler moved a distance of 8.4 m up the incline? at what rate is the force of the rope doing work on the skier when the rope moves with a speed of (b) 0.69 m/s and (c) 2.2 m/s?
Answers: 1


Physics, 22.06.2019 12:10
Consider a one meter long horizontal pipe with a constant 100 cm^2 cross sectional area. water flows rightward into the pipe at x = 0 with flow velocity 02m/sec at every point within the pipe intake area. at x=1, the rightward flow rate is 0.192 m/sec. assume the water is a conserved quantity in the pipe, so there must be a leak (a sink) somewhere in the pipe. 1. compute net volumetric flow of the source if the system to be in equilibrium. 2. now assume the pipe in the problem has no leaks. compute the net volumetric rate of change for the system.
Answers: 3
You know the right answer?
One might be tempted to say that exothermic processes are always spontaneous since the system is emi...
Questions


Biology, 24.08.2019 10:30








Geography, 24.08.2019 10:30

Mathematics, 24.08.2019 10:30

History, 24.08.2019 10:30


History, 24.08.2019 10:30


Mathematics, 24.08.2019 10:30

Mathematics, 24.08.2019 10:30



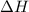 = 17 KJ/mole = 17000 J/mole
= 17 KJ/mole = 17000 J/mole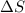 = 50 J/mole.K
= 50 J/mole.K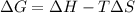
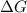 is negative or we can say that the value of
is negative or we can say that the value of