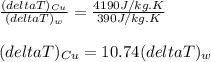Physics, 18.12.2019 03:31 7thaohstudent
A1.0 kg piece of copper with a specific heat of ccu=390j/(kg⋅k) is placed in 1.0 kg of water with a specific heat of cw=4190j/(kg⋅k). the copper and water are initially at different temperatures. after a sufficiently long time, the copper and water come to a final equilibrium temperature. part a which of the following statements is correct concerning the temperature changes of both substances? (ignore the signs of the temperature changes in your answer.) which of the following statements is correct concerning the temperature changes of both substances? (ignore the signs of the temperature changes in your answer.) the temperature change of the copper is equal to the temperature change of the water. the temperature change of the water is greater than the temperature change of the copper. the temperature change of the copper is greater than the temperature change of the water.

Answers: 2


Another question on Physics

Physics, 21.06.2019 21:00
State the right hand rule of the direction of magnetic field in a conductor carrying current
Answers: 1

Physics, 22.06.2019 04:00
What is the scientific definition of energy that relates to work
Answers: 1

Physics, 22.06.2019 07:20
If the ama of the inclined plane below is 2, calculate the ima and efficiency. ima = efficiency =
Answers: 1

Physics, 22.06.2019 10:30
The freezing and boiling point of a substance changes as the air pressure around it changes. for example, at a lower air pressure (higher altitude) it is easier for water molecules to escape from liquid into the air. in a high altitude city such as denver, colorado compared to a sea-level city such as houston, texas, water
Answers: 2
You know the right answer?
A1.0 kg piece of copper with a specific heat of ccu=390j/(kg⋅k) is placed in 1.0 kg of water with a...
Questions

Mathematics, 21.08.2020 19:01




Mathematics, 21.08.2020 19:01


Mathematics, 21.08.2020 19:01










Chemistry, 21.08.2020 19:01