At 250 °c, the equilibrium constant kp for the reaction pcl5 (g) pcl3 (g) + cl2 (g) is 1.80. sufficient pcl5 is put into a reaction vessel to give an initial pressure of 2.74 atm at 250 °c. calculate the pressure of pcl5 after the system has reached equilibrium.
a. 1.50 atm
b. 1.24 atm
c. 4.24 atm
d. 0.94 atm
e. 1.12 atm

Answers: 3


Another question on Physics


Physics, 22.06.2019 18:30
Against his financial advisor's advice, frank has decided to invest his money in some risky stocks because he once made quite a bit of money in the stock market. his decision illustrates a. the representativeness heuristic b. overconfidence c. the availability heuristic d. confirmation bias select the best answer from the choices provided
Answers: 3

Physics, 22.06.2019 18:30
Examples of states of consciousness include a. daydreaming b. dreaming during sleep c. hypnosis d. all of the above
Answers: 2

Physics, 22.06.2019 22:00
In the united states, tornadoes generally occur because of the freezing of ocean water underwater earthquakes meeting of cool and warm air masses shifting of warm and cool ocean currents
Answers: 2
You know the right answer?
At 250 °c, the equilibrium constant kp for the reaction pcl5 (g) pcl3 (g) + cl2 (g) is 1.80. suffici...
Questions


English, 18.12.2020 18:40






Mathematics, 18.12.2020 18:40


Mathematics, 18.12.2020 18:40

English, 18.12.2020 18:40


Health, 18.12.2020 18:40




Mathematics, 18.12.2020 18:40

Arts, 18.12.2020 18:40

Mathematics, 18.12.2020 18:40

Mathematics, 18.12.2020 18:40

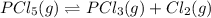
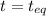 2.74-x x x
2.74-x x x
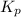 for the given reaction follows:
for the given reaction follows:
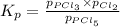
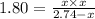
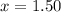
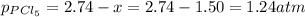
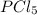 at equilibrium is 1.24 atm
at equilibrium is 1.24 atm