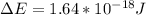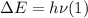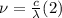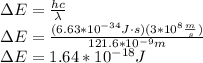Physics, 10.12.2019 06:31 alyssamaize
When an electron falls from a higher to a lower energy level in an atom, the photon released has a wavelength of 121.6 nm. what is the energy difference between the two energy levels, in j?

Answers: 3


Another question on Physics

Physics, 22.06.2019 11:50
Amoving electron has kinetic energy k1. after a net amount of work w has been done on it, the electron is moving one-quarter as fast in the opposite direction. (a) find w in terms of k1. (b) does your answer depend on the final direction of the electron's motion?
Answers: 2

Physics, 22.06.2019 12:10
Energy flows from the producer level to the level. is called
Answers: 1

Physics, 22.06.2019 18:00
A4.0-cm tall light bulb is placed a distance of 32.0 cm from a concave mirror having a focal length of 16.0 cm. determine the image distance
Answers: 1

Physics, 22.06.2019 19:30
Because atoms of elements in the same group of thbecause atoms of elements in the same group of the periodic table have the same number of neutrons, they have similar properties. select the best answer from the choices provided t fe periodic table have the same number of neutrons, they have similar properties. select the best answer from the choices provided t f
Answers: 1
You know the right answer?
When an electron falls from a higher to a lower energy level in an atom, the photon released has a w...
Questions




English, 10.10.2020 17:01


Biology, 10.10.2020 17:01

Mathematics, 10.10.2020 17:01




Mathematics, 10.10.2020 17:01



Mathematics, 10.10.2020 17:01


Biology, 10.10.2020 17:01

Mathematics, 10.10.2020 17:01


Law, 10.10.2020 17:01

Chemistry, 10.10.2020 17:01