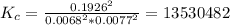Physics, 10.12.2019 02:31 mooncake9090
Areaction mixture in a 3.67 l flask at a certain temperature initially contains 0.763 g h2 and 96.9 g i2, at equilibrium, the flask contains 90.4 g hi. calculate the equilibrium constant (kc) for the reaction at this temperature.

Answers: 2


Another question on Physics

Physics, 21.06.2019 18:00
Assuming weightless pulleys and 100% efficiency, what is the minimum input force required to lift a 120 n weight using a single fixed pulley?
Answers: 2

Physics, 21.06.2019 19:10
Natural forces that can alter ecosystems include seasons and climate changes. t or f
Answers: 1

Physics, 22.06.2019 00:30
During spring semester at mit, residents of the parallel buildings of the east campus dorms battle one another with large catapults that are made with surgical hose mounted on a window frame. a balloon filled with dyed water is placed in a pouch attached to the hose, which is then stretched through the width of the room. assume that the stretching of the hose obeys hooke's law with a spring constant of 89.0 n/m. if the hose is stretched by 5.80 m and then released, how much work does the force from the hose do on the balloon in the pouch by the time the hose reaches its relaxed length? unitst 3 number-1497 the tolerance is +/-5% open show work click if you would like to show work for this question:
Answers: 2

Physics, 22.06.2019 08:30
What properties of a moving object are used in determining the object's energy of motion
Answers: 2
You know the right answer?
Areaction mixture in a 3.67 l flask at a certain temperature initially contains 0.763 g h2 and 96.9...
Questions

Business, 13.07.2019 14:20



Mathematics, 13.07.2019 14:20


Physics, 13.07.2019 14:20

Mathematics, 13.07.2019 14:20

History, 13.07.2019 14:20


English, 13.07.2019 14:20

Geography, 13.07.2019 14:20




Mathematics, 13.07.2019 14:20