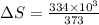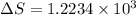Physics, 09.12.2019 20:31 cassiemyers60
Calculate the change in entropy when 1.00 kg of water at 100 degree c is vaporized and converted to steam at 100 degree c. assume that the heat of vaporization of water is 2256 times 103 j/kg. calculate the change in entropy when 1.00 kg of ice is melted at 0 degree c. assume that the heat of fusion of water is lf = 3.34 times 105j/kg. is the change entropy greater for melting or for vaporization? the change entropy greater for melting the change entropy greater for vaporization

Answers: 3


Another question on Physics

Physics, 21.06.2019 18:30
A10.0 kg cart and a 15kg cart are locked together with a compressed spring between them. they are then released so that the spring pushes the two carts apart. the 10.0 kg cart is moving at 4.5 m/s afterward. how fast is the 15kg cart moving? 3.0 m/s 2.0 m/s 4.5 m/s 5.0 m/s
Answers: 1

Physics, 22.06.2019 19:30
Acamcorder has a power rating of 20 watts. if the output voltage from its battery is 9 volts, what current does it use?
Answers: 2

Physics, 22.06.2019 21:30
Aperson touches a large chunk of ice with their hand and remarks, “this is making me cold.” explain what this person is feeling. is the ice transferring “cold” to the person? is there a heat transfer occurring? explain.
Answers: 1

You know the right answer?
Calculate the change in entropy when 1.00 kg of water at 100 degree c is vaporized and converted to...
Questions





Mathematics, 24.05.2021 20:40

Mathematics, 24.05.2021 20:40



Mathematics, 24.05.2021 20:40

Spanish, 24.05.2021 20:40





Mathematics, 24.05.2021 20:40

History, 24.05.2021 20:40

History, 24.05.2021 20:40

Mathematics, 24.05.2021 20:40

Mathematics, 24.05.2021 20:40

Mathematics, 24.05.2021 20:40

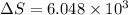
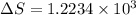
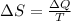
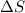 is change of entropy,
is change of entropy, 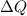 is change of heat and T is absolute temperature in kelvin
is change of heat and T is absolute temperature in kelvin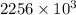 J/kg.
J/kg.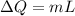
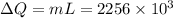
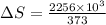
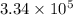 J/kg.
J/kg.![3.34 \times 10^{5}=334 \times 10^{3} J/kg.[tex]\Delta Q = mL = 334 \times 10^{3}](/tpl/images/0410/3794/17bda.png)