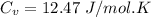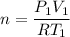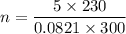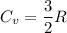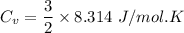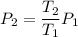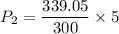Physics, 07.12.2019 03:31 ggpro4life3000
An ideal monatomic gas is contained in a vessel of constant volume 0.230 m3. the initial temperature and pressure of the gas are 300 k and 5.00 atm, respectively. the goal of this problem is to find the temperature and pressure of the gas after 23.0 kj of thermal energy is supplied to the gas.
(a) use the ideal gas law and initial conditions to calculate the number of moles of gas in the vessel.
mol
(b) find the specific heat of the gas.
j/k
(c) what is the work done by the gas during this process?
kj
(d) use the first law of thermodynamics to find the change in internal energy of the gas.
kj
(e) find the change in temperature of the gas.
k
(f) calculate the final temperature of the gas.
k
(g) use the ideal gas expression to find the final pressure of the gas.
atm

Answers: 1


Another question on Physics

Physics, 22.06.2019 02:30
Which of the following are properties of mechanical waves? check all that apply ⭕️ particles of the medium move back and fourth, but do not move with the wave. ⭕️ the particles of the medium always move parallel to the wave motion. ⭕️ wave motion begins with a disturbance in the medium. ⭕️ waves transport energy from a source outward, away from the source.
Answers: 2

Physics, 22.06.2019 09:30
Which of these is not a possible type of energy transformation? a. electrical energy into light energy b. sound energy into nuclear energy c. potential energy into kinetic energy d. kinetic energy into mechanical energy
Answers: 1


Physics, 22.06.2019 11:20
The ultracentrifuge is an important tool for separating and analyzing proteins. because of the enormous centripetal accelerations, the centrifuge must be carefully balanced, with each sample matched by a sample of identical mass on the opposite side. any difference in the masses of opposing samples creates a net force on the shaft of the rotor, potentially leading to a catastrophic failure of the apparatus. suppose a scientist makes a slight error in sample preparation and one sample has a mass 10 mg larger than the opposing sample. if the samples are 12 cm from the axis of the rotor and the ultracentrifuge spins at 70,000 rpm, what is the magnitude of the net force on the rotor due to the unbalanced samples? ( be thorough on your answer)
Answers: 3
You know the right answer?
An ideal monatomic gas is contained in a vessel of constant volume 0.230 m3. the initial temperature...
Questions



Mathematics, 10.10.2019 14:10

Chemistry, 10.10.2019 14:10

History, 10.10.2019 14:10




English, 10.10.2019 14:10




English, 10.10.2019 14:10

History, 10.10.2019 14:10

Biology, 10.10.2019 14:10




Mathematics, 10.10.2019 14:10