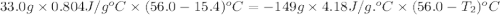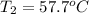Physics, 06.12.2019 17:31 sergiog6833
Ideally, when a thermometer is used to measure the temperature of an object, the temperature of the object itself should not change. however, if a significant amount of heat flows from the object to the thermometer, the temperature will change. a thermometer has a mass of 33.0 g, a specific heat capacity of c = 804 j/(kg c°), and a temperature of 15.4 °c. it is immersed in 149 g of water, and the final temperature of the water and thermometer is 56.0 °c. what was the temperature of the water in degrees celsius before the insertion of the thermometer?

Answers: 2


Another question on Physics

Physics, 22.06.2019 18:50
8.29 two streams containing pyridine and acetic acid at 25°c are mixed and fed into a heat exchanger. due to the heat-of-mixing effect, it is desired to reduce the temperature after mixing to 25°c using a stream of chilled ethylene glycol as indicated in the diagram. calculate the mass flow rate of ethylene glycol needed. the heat capacity of ethylene glycol at these conditions is approximately 2.8 kj/(kg k), and the enthalpy change of mixing (δmixh) is given below.
Answers: 3

Physics, 22.06.2019 20:30
Which statement best describes the direction of the buoyant force on any object? a. opposite the force of gravity b. in the same direction as the weight c. in the direction of motion of the object d. opposite the direction of motion of the object
Answers: 1

Physics, 22.06.2019 23:50
The star nearest to our sun is proxima centauri, at a distance of 4.3 light-years from the sun. how far away, in km, is proxima centauri from the sun?
Answers: 3

Physics, 23.06.2019 00:50
Which of the following is not an appropriate unit for acceleration? (kg/12 mihr? m/min/sec cm/30c?
Answers: 2
You know the right answer?
Ideally, when a thermometer is used to measure the temperature of an object, the temperature of the...
Questions

Health, 19.08.2019 09:30

Mathematics, 19.08.2019 09:30


English, 19.08.2019 09:30


Physics, 19.08.2019 09:30

History, 19.08.2019 09:30



History, 19.08.2019 09:30

Social Studies, 19.08.2019 09:30

Mathematics, 19.08.2019 09:30

Mathematics, 19.08.2019 09:30



History, 19.08.2019 09:30

History, 19.08.2019 09:30


Mathematics, 19.08.2019 09:30

Mathematics, 19.08.2019 09:30

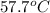
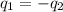
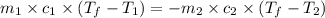
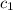 = specific heat of thermometer =
= specific heat of thermometer = 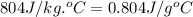
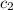 = specific heat of water =
= specific heat of water = 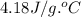
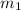 = mass of thermometer = 33.0 g
= mass of thermometer = 33.0 g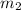 = mass of water = 149 g
= mass of water = 149 g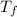 = final temperature =
= final temperature = 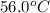
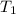 = initial temperature of thermometer =
= initial temperature of thermometer = 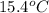
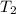 = initial temperature of water = ?
= initial temperature of water = ?