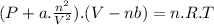Answers: 3


Another question on Physics


Physics, 22.06.2019 15:30
The voltage applied across a given parallel-plate capacitor is doubled. how is the energy stored in the capacitor affected?
Answers: 2

Physics, 22.06.2019 19:00
The built in flash in a compact camera is usally capable of giving correct exsposure for distance up to how many meters?
Answers: 1

Physics, 22.06.2019 19:30
In this thread, i would like you to comment on the nature of light and how operation of telescopes. light has a duality of a particle and a wave; which one affects your life? also how does light interact with optics inside telescopic systems? answer these questions in two paragraphs. then respond the another students response.
Answers: 1
You know the right answer?
A9.800 mol sample of nitrogen gas is maintained in a 0.8166 l container at 301.8 k. what is the pres...
Questions

History, 14.07.2019 11:30


Mathematics, 14.07.2019 11:30

Chemistry, 14.07.2019 11:30




Biology, 14.07.2019 11:30


Mathematics, 14.07.2019 11:30


Social Studies, 14.07.2019 11:30



Biology, 14.07.2019 11:30




Mathematics, 14.07.2019 11:30