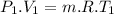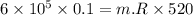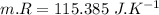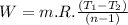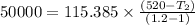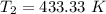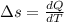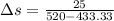Nitrogen (n2) undergoes an internally reversible process from 6 bar, 247°c during which pν1.2 = constant. the initial volume is 0.1 m3 and the work for the process is 50 kj. assuming ideal gas behavior, and neglecting kinetic and potential energy effects, determine heat transfer, in kj, and the entropy change, in kj/k.

Answers: 2


Another question on Physics

Physics, 21.06.2019 15:30
What defines the mass number of an isotope? a. the sum of the neutrons and protons b. the sum of the neutrons and electrons c. the number of neutrons d. the number of protons
Answers: 1

Physics, 21.06.2019 19:30
Asample of a gaseous substance changes into liquid. this process is called (blank) and it takes place when heat is (blank) a gaseous system
Answers: 2

Physics, 22.06.2019 18:00
According to newton’s law of universal gravitation, which statements are true? as we move to higher altitudes, the force of gravity on us decreases. as we move to higher altitudes, the force of gravity on us increases. as we gain mass, the force of gravity on us decreases. as we gain mass, the force of gravity on us increases. as we move faster, the force of gravity on us increases.
Answers: 2

Physics, 23.06.2019 01:00
Which pair of quantities includes one quantity that increases as the other decreases during simple harmonic motion?
Answers: 2
You know the right answer?
Nitrogen (n2) undergoes an internally reversible process from 6 bar, 247°c during which pν1.2 = cons...
Questions


Mathematics, 11.10.2019 04:30



Engineering, 11.10.2019 04:30


Health, 11.10.2019 04:30







Computers and Technology, 11.10.2019 04:30


Business, 11.10.2019 04:30


Mathematics, 11.10.2019 04:30


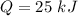
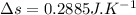
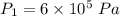 initial temperature,
initial temperature, 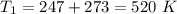 polytropic index,
polytropic index, 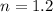 initial volume,
initial volume, 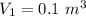 work done in the process,
work done in the process, 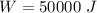
![Q=W[\frac{\gamma -n}{\gamma-1} ]](/tpl/images/0402/4036/a3071.png)
![Q=50\times[\frac{1.4-1.2}{1.4-1} ]](/tpl/images/0402/4036/3ed0d.png)