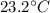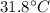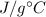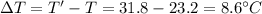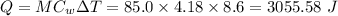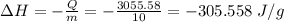In a coffee-cup calorimeter experiment, 10.00 g of a soluble ionic compound was added to the calorimeter contained 75.0 g h2o initially at 23.2°c. the final temperature of the solution was 31.8°c. what was the change in enthalpy for the dissolution of this compound?

Answers: 1


Another question on Physics


Physics, 22.06.2019 11:00
If a simple machine reduces the strength of a force, what must be increased?
Answers: 3

Physics, 22.06.2019 13:50
9.98 kg of r-134a at 300 kpa fills a rigid container whose volume is 14 l. determine the temperature and total enthalpy in the container. the container is now heated until the pressure is 600 kpa. determine the temperature and total enthalpy when the heating is completed. use data from the steam tables.
Answers: 1

Physics, 22.06.2019 14:10
Match these items. 1. coulombs __force 2. ohms __emf 3. centimeters __resistance 4. newtons __charge 5. volts __length
Answers: 1
You know the right answer?
In a coffee-cup calorimeter experiment, 10.00 g of a soluble ionic compound was added to the calorim...
Questions

English, 19.02.2020 11:57


Mathematics, 19.02.2020 11:58



Chemistry, 19.02.2020 12:00


Law, 19.02.2020 12:01

Mathematics, 19.02.2020 12:01


History, 19.02.2020 12:04

Mathematics, 19.02.2020 12:05

Mathematics, 19.02.2020 12:08


History, 19.02.2020 12:09

Mathematics, 19.02.2020 12:09

Mathematics, 19.02.2020 12:10