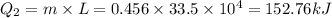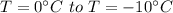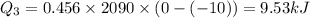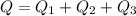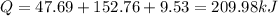Physics, 04.12.2019 00:31 amandasantiago2001
How much heat must be removed from 456 g of water at 25.0°c to change it into ice at - 10.0°c ? the specific heat of ice is 2090 j/(kg. k) and the latent heat of fusion of water is 33.5 x 10^4 j/kg .

Answers: 3


Another question on Physics

Physics, 22.06.2019 04:10
You will be galileo perform the experiment to determine if objects with different mass fall at the same, or different, rates in the air and in a vacuum. (refer to the walk-through video to locate the online lab within the online textbook).
Answers: 2

Physics, 22.06.2019 13:00
Nacidified solution was electrolyzed using copper electrodes. a constant current of 1.18 a caused the anode to lose 0.584 g after 1.52 ✕ 103 s. given that the charge of an electron is 1.6022 ✕ 10−19 c, calculate avogadro's number. assume that copper is oxidized to cu2+ ions.
Answers: 1


Physics, 23.06.2019 00:30
Asmall electric heater, immersed in 80g of water in a calorimeter of 40j/k raises the temperature from 10.5 degree celsius to 25 degree celsius in 12 minutes. using the same apparatus with 50g of oil instead of water, it is found that the temperature of oil rises from 7 degree celsius to 27 degree celsius in 10 minutes. find specific heat capacity of the oil.
Answers: 2
You know the right answer?
How much heat must be removed from 456 g of water at 25.0°c to change it into ice at - 10.0°c ? the...
Questions







Mathematics, 02.10.2020 14:01

Medicine, 02.10.2020 14:01

History, 02.10.2020 14:01

Health, 02.10.2020 14:01






Mathematics, 02.10.2020 14:01

History, 02.10.2020 14:01



Mathematics, 02.10.2020 14:01

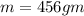
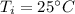
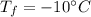
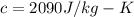
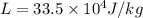
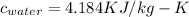
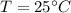 to
to 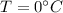
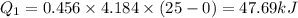
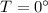 to ice at
to ice at