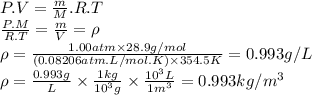Physics, 29.11.2019 02:31 garcser257278
If the molecular weight of air is 28.9, what is the density of air at atmospheric pressure and a temperature of 354.5 k? 1 atm = 1.013 × 105 n/m2 , the mass of a proton is 1.67262 × 10−27 kg , avogadro’s number is 6.02214 × 1023 mol−1 and k = 1.38065 × 10−23 n · m/k . answer in units of kg/m3

Answers: 3


Another question on Physics

Physics, 22.06.2019 01:00
Avehicle has an oil leak that is causing the entire oil pan to be wet, but inspection reveals no exact source after cleaning off the oil residue. technician a says to install a fluorescent dye in the crankcase and operate the engine, then re-inspect for leaks with a special light (black light). technician b says the oil leak may be coming from a source at the top of the engine, such as a valve cover gasket. who is correct?
Answers: 1


Physics, 22.06.2019 08:50
You are given a vector a = 125i and an unknown vector b that is perpendicular to a. the cross-product of these two vectors is a × b = 98k. what is the y-component of vector b?
Answers: 1

Physics, 23.06.2019 02:00
How does the strength of the electrostatic force between an electron and a proton compare to the strength of the gravitational force between them?
Answers: 1
You know the right answer?
If the molecular weight of air is 28.9, what is the density of air at atmospheric pressure and a tem...
Questions



Advanced Placement (AP), 24.02.2021 06:50



Mathematics, 24.02.2021 06:50





Mathematics, 24.02.2021 06:50


History, 24.02.2021 06:50

Spanish, 24.02.2021 06:50


Mathematics, 24.02.2021 06:50


Biology, 24.02.2021 06:50


Mathematics, 24.02.2021 06:50