
Physics, 27.11.2019 06:31 yomamafart7
A(10.0+a) g ice cube at -15.0oc is placed in (125+b) g of water at 48.0oc.
find the final temperature of the system when equilibrium is reached.
ignore the heat capacity of the container and assume this is in a calorimeter, i. e. the system is thermally insulated from the surroundings.
give your answer in oc with 3 significant figures.

Answers: 1


Another question on Physics

Physics, 21.06.2019 23:20
Asmall, positively charged ball is moved close to a large, positively charged ball. which describes how the small ball likely responds when it is released? it will move toward the large ball because like charges repel. it will move toward the large ball because like charges attract. it will move away from the large ball because like charges repel. it will move away from the large ball because like charges attract.
Answers: 3

Physics, 22.06.2019 09:00
One form of energy can be another type of energy. a. created to form b. transformed into c. destroyed and then created to form
Answers: 1

Physics, 22.06.2019 09:30
True or false graphs must include scales that increase by the same amount
Answers: 3

You know the right answer?
A(10.0+a) g ice cube at -15.0oc is placed in (125+b) g of water at 48.0oc.
find the fin...
find the fin...
Questions








Physics, 02.11.2020 16:50






History, 02.11.2020 16:50


Chemistry, 02.11.2020 16:50




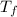 = 38.0° C
= 38.0° C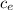 ΔT
ΔT 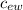 (water) = 4186 J / kg ºC
(water) = 4186 J / kg ºC 

