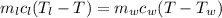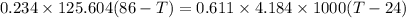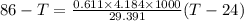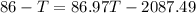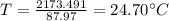Physics, 27.11.2019 06:31 fannyrivera321
A234.0 g piece of lead is heated to 86.0oc and then dropped into a calorimeter containing 611.0 g of water that initally is at 24.0oc. neglecting the heat capacity of the container, find the final equilibrium temperature (in oc) of the lead and water.

Answers: 2


Another question on Physics

Physics, 22.06.2019 14:30
When the displacement of a mass on a spring is 12a the half of the amplitude, what fraction of the mechanical energy is kinetic energy? at what displacement, as a fraction of a, is the mechanical energy half kinetic and half potential?
Answers: 3

Physics, 22.06.2019 17:10
Which statement best describes the superposition principle? a.) if two in-phase waves arrive simultaneously at a point, their amplitudes add up b.) if two out-of-phase waves arrive simultaneously at a point, their amplitudes add up c.) if two in-phase waves arrive at a point one after another, their amplitudes add up d.) if two out-of-phase waves arrive at a point one after another, their amplitudes adds up
Answers: 2


Physics, 22.06.2019 21:00
The velocity of a car traveling in a straight line increases from 0 meters/second to 30meters/second in 8 seconds. what is the average acceleration of the car?
Answers: 1
You know the right answer?
A234.0 g piece of lead is heated to 86.0oc and then dropped into a calorimeter containing 611.0 g of...
Questions



Mathematics, 18.03.2021 01:20



Mathematics, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20


History, 18.03.2021 01:20



Mathematics, 18.03.2021 01:20


Geography, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20

English, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20

Social Studies, 18.03.2021 01:20


Biology, 18.03.2021 01:20

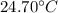
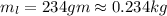
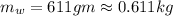
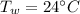
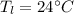
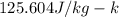 and
and 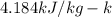 respectively
respectively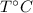 be the final temperature of the system
be the final temperature of the system