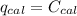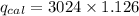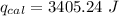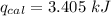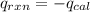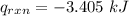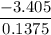Physics, 27.11.2019 01:31 CyberSongWriter
When 0.1375 g of solid magnesium is burned in a constant-volume bomb calorimeter, the temperature increases by 1.126°c. the heat capacity of the bomb calorimeter, determined in a separate experiment, is 3024 j/°c. calculate the heat given off by the burning magnesium in kj/mol.

Answers: 1


Another question on Physics

Physics, 22.06.2019 07:30
Examine the nuclear reacti why is this classified as a nuclear reaction rather than a chemical reaction? it is not balanced. a new compound is formed. a change has occurred in a nucleus. a new element has been formed.
Answers: 2

Physics, 22.06.2019 11:20
If the radius of curvature of the cornea is 0.75 cm when the eye is focusing on an object 36.0 cm from the cornea vertex and the indexes of refraction are as described before, what is the distance from the cornea vertex to the retina? express your answer to two significant
Answers: 3


Physics, 22.06.2019 16:30
The air in an automobile tire with a volume of 2.60 ft3 is at 70°f and 21 psig. determine the amount of air that must be added to raise the pressure to the recommended value of 30 psig. assume the atmospheric pressure to be 14.6 psia and the temperature and the volume to remain constant. the gas constant of air is ru = 53.34ft⋅lbflbm⋅r(1 psia144 lbf/ft2) = 0.3704 psia⋅ft3lbm⋅r the amount of air that must be added to raise the pressure is lbm
Answers: 3
You know the right answer?
When 0.1375 g of solid magnesium is burned in a constant-volume bomb calorimeter, the temperature in...
Questions


English, 29.05.2021 01:00

World Languages, 29.05.2021 01:00





Mathematics, 29.05.2021 01:00






Mathematics, 29.05.2021 01:00



Mathematics, 29.05.2021 01:00

Geography, 29.05.2021 01:00


Social Studies, 29.05.2021 01:00