
In the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used to determine the specific heat of a solid, or to measure the energy of a solution phase reaction. a student heats 69.12 grams of tungsten to 98.93 °c and then drops it into a cup containing 85.45 grams of water at 23.82 °c. she measures the final temperature to be 25.63 °c. the heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.56 j/°c. assuming that no heat is lost to the surroundings calculate the specific heat of tungsten.

Answers: 1


Another question on Physics

Physics, 21.06.2019 21:50
What is the length x of the side of the triangle below? (hint: use the cosine function)
Answers: 1

Physics, 22.06.2019 17:00
How much energy is supplied to each coulomb of charge that flows through a 12-v battery?
Answers: 1

Physics, 22.06.2019 22:00
Aviolently rotating column of air is formed around a region of extremely low air pressure above land. which of these weather conditions is most likely to follow? blizzard cyclone tornado hurricane
Answers: 1

Physics, 23.06.2019 00:30
Discuss the difference between thermal energy and heat. (terrance) terrance, egolf, r., congdon, donald. physical science student text, 5th edition. bju press, 2014. vitalbook file.
Answers: 1
You know the right answer?
In the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used t...
Questions

History, 24.09.2019 03:50

Biology, 24.09.2019 03:50

Business, 24.09.2019 03:50

Biology, 24.09.2019 03:50

Computers and Technology, 24.09.2019 03:50

English, 24.09.2019 03:50


Physics, 24.09.2019 03:50

History, 24.09.2019 03:50





Chemistry, 24.09.2019 03:50


History, 24.09.2019 03:50



Biology, 24.09.2019 03:50

Mathematics, 24.09.2019 03:50

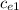 = 128.3 J / kg ° C
= 128.3 J / kg ° C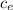 ΔT
ΔT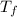 ) = (m₂
) = (m₂ 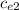 + C) (
+ C) (

