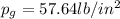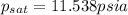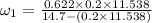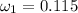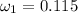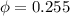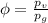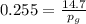Physics, 19.11.2019 06:31 fqjenfiq6959
Amixture of nitrogen and water vapor at 200of, 1 atm has the molar analysis of 80% n2, 20% water vapor. a) if the mixture is cooled at constant pressure, determine the temperature, in of, at which water vapor begins to condense. b) if the mixture is compressed at constant temperature, determine the pressure, in atm, at which water vapor begins to condense.

Answers: 3


Another question on Physics

Physics, 22.06.2019 05:00
Which car has the most kinetic energy? a. a car of mass 1000 kg with a speed of 3 m/s b. a car of mass 2000 kg with speed 7 m/s c. a car of mass 1000 kg with speed 7 m/s d. a car of mass 2000 kg with speed 3 m/s
Answers: 1

Physics, 22.06.2019 19:20
On a hot saturday morning while people are working inside, the air conditioner keeps the temperature inside the building at 22degreesc. at noon the air conditioner is turned off, and the people go home. the temperature outside is a constant 33degreesc for the rest of the afternoon. if the time constant for the building is 4 hr, what will be the temperature inside the building at 4 : 00 font size decreased by 2 upper p . font size decreased by 2 upper m .? at 6 : 00 font size decreased by 2 upper p . font size decreased by 2 upper m .? when will the temperature inside the building reach 24degreesc? 324281
Answers: 3

Physics, 22.06.2019 22:00
Aspaceship starting from a resting position accelerates at a constant rate of 9.8 meters per second per second. how far will the spaceship travel if its final speed is 1 percent the speed of light (300,000,000 m/s)? a. 3.1 × 106 m b. 2.94 × 108 m c. 7.8 × 1010 m d. 4.6 × 1013 m
Answers: 1

Physics, 22.06.2019 23:50
What is part of a line has one endpoint and continues in one direction?
Answers: 1
You know the right answer?
Amixture of nitrogen and water vapor at 200of, 1 atm has the molar analysis of 80% n2, 20% water vap...
Questions



English, 11.11.2020 21:20

Business, 11.11.2020 21:20



Mathematics, 11.11.2020 21:20




Spanish, 11.11.2020 21:20

Mathematics, 11.11.2020 21:20

Mathematics, 11.11.2020 21:20



Mathematics, 11.11.2020 21:20

Mathematics, 11.11.2020 21:20


Mathematics, 11.11.2020 21:20