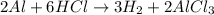Physics, 11.11.2019 08:31 caromaybelline71
How does the law of conservation of mass apply to this reaction: al + 3hcl → h2 + alcl3 ?
hydrogen and chlorine need to be balanced. there is an equal amount of aluminum on each side.
only the hydrogen needs to be balanced. there are equal numbers of aluminum and chlorine.
the law of conservation of mass has already been applied. there is an equal number of each element on both sides of the equation.
the equation needs to be balanced. there are fewer hydrogen atoms in the equation than aluminum or chlorine.

Answers: 2


Another question on Physics

Physics, 21.06.2019 19:30
A500g object falls off a cliff and losers 100 j from its gravitational potential energy store. if the gravitational field strength g=9.8n/kg, how high is the cliff?
Answers: 1


Physics, 22.06.2019 07:20
What is the magnitude of the acceleration vector which causes a particle to move from velocity −5i−2j m/s to −6i+ 7j m/s in 8 seconds. answer in m/s.
Answers: 3

Physics, 22.06.2019 15:30
What is a view of science and psychology that says the value of knowledge depends on its usefulness? a. pragmatism b. psychotherapy c. physiology
Answers: 2
You know the right answer?
How does the law of conservation of mass apply to this reaction: al + 3hcl → h2 + alcl3 ?
Questions

Social Studies, 03.12.2021 01:00

Physics, 03.12.2021 01:00

Mathematics, 03.12.2021 01:00

Physics, 03.12.2021 01:00

Mathematics, 03.12.2021 01:00

Mathematics, 03.12.2021 01:00

Computers and Technology, 03.12.2021 01:00



Mathematics, 03.12.2021 01:00


Biology, 03.12.2021 01:00



Mathematics, 03.12.2021 01:00


Mathematics, 03.12.2021 01:00

History, 03.12.2021 01:00

Computers and Technology, 03.12.2021 01:00

Social Studies, 03.12.2021 01:00