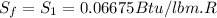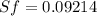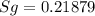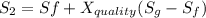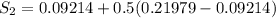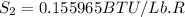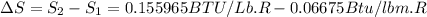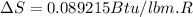Physics, 10.11.2019 06:31 GFJNIN9858
One lb of refrigerant 134a contained within a piston–cylinder assembly undergoes a process from a state where the temperature is 60f and the refrigerant is saturated liquid to a state where the pressure is 140 lbf/in 2 and quality is 50%. determine the change in specific entropy of the refrigerant, in btu/lbr. can this process be accomplished adiabatically?

Answers: 1


Another question on Physics

Physics, 21.06.2019 19:30
Describe the path of a ray that approaches a mirror parallel to the principal axis.
Answers: 2

Physics, 22.06.2019 00:30
Imagine you work as a consultant, and your daily job involves listening to the constructive criticism of others. as part of your job, you must listen to opposing viewpoints to make important decisions. in this activity, you are a consulting dam engineer. you must make a decision about four different dams; should they be repaired, taken down, or left alone? you will listen to contrasting opinions about what should be done with the dams before you make the final decision. as the consulting dam engineer, what do you decide to do with this dam? explain your reasoning. by making your decision, did you support the opinions of the mayor and/or the dam safety official? why or why not? (site 1)
Answers: 1


Physics, 22.06.2019 09:30
In a heat engine if 1000 j of heat enters the system the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2000 j? 1. write the equation 2.list out your known variables 3.plug the numbers into the equations 4.solve 5.write your solution statement that includes initial energy and final
Answers: 3
You know the right answer?
One lb of refrigerant 134a contained within a piston–cylinder assembly undergoes a process from a st...
Questions


Mathematics, 20.10.2019 21:20


Chemistry, 20.10.2019 21:20

Chemistry, 20.10.2019 21:20

Physics, 20.10.2019 21:20

Social Studies, 20.10.2019 21:20




History, 20.10.2019 21:20



History, 20.10.2019 21:20





Mathematics, 20.10.2019 21:20

Business, 20.10.2019 21:20