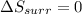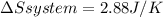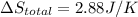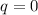Physics, 07.11.2019 04:31 Karolina0304
Calculate the change in the entropies of the system and the surroundings, and the total change in entropy, when a sample of nitrogen gas of mass 14 g at 298 k and 1.00 bar doubles its volume in (a) an isothermal reversible expansion, (b) an isothermal irreversible expansion against pex = 0, and (c) an adiabatic reversible expansion.

Answers: 3


Another question on Physics

Physics, 22.06.2019 05:50
There are two routes similar in terrain; however, one route has an incline of approximately 28 degrees, and you must decide on which route to take. what is the maximum road grade that your fully loaded ecv can climb?
Answers: 2

Physics, 22.06.2019 14:00
Agraduated cylinder contains 63.0 ml of water. a piece of gold, which has a density of 19.3 g/ cm3, is added to the water and the volume goes up to 64.5 ml. calculate the mass in grams of the gold that was added to the water. explain how you got your answer.
Answers: 3


Physics, 23.06.2019 00:00
Which one of the following represents the reduced forms of the two major electron carriers? nadh and fadh2 nad+ and fadh2 nad+ and fad nadh and fad
Answers: 3
You know the right answer?
Calculate the change in the entropies of the system and the surroundings, and the total change in en...
Questions


Mathematics, 15.01.2021 01:00



Mathematics, 15.01.2021 01:00

History, 15.01.2021 01:00


Biology, 15.01.2021 01:00

Mathematics, 15.01.2021 01:00

Biology, 15.01.2021 01:00


English, 15.01.2021 01:00

Mathematics, 15.01.2021 01:00


Mathematics, 15.01.2021 01:00


Health, 15.01.2021 01:00

Biology, 15.01.2021 01:00

Advanced Placement (AP), 15.01.2021 01:00

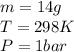
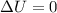
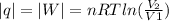
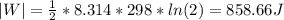
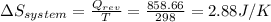
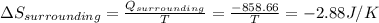
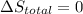
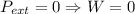
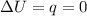 , since isothermal
, since isothermal