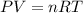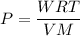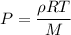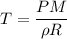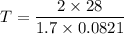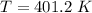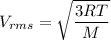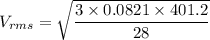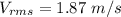Acontainer of gas molecules is at a pressure of 2 atm and has amass density of 1.7 grams per liter. all of the molecules in thecontainer are diatomic nitrogen molecules with an atomic weight of28 grams per mole. what is the typical speed of the nitrogenmolecules in the container? here we define the typical speed to bethe root-mean-square velocity (rms velocity = vrms) ofthe center of mass of the molecule

Answers: 3


Another question on Physics

Physics, 22.06.2019 02:30
Acup of coffee sits on the dashboard of an automobile. the car goes around a sharp curve. even though you hold the cup still, coffee still splashes out. this can best be explained due to a) density. b) friction. c) gravity. d) inertia.
Answers: 2

Physics, 22.06.2019 23:50
What is part of a line has one endpoint and continues in one direction?
Answers: 1

Physics, 23.06.2019 01:30
The first law of thermodynamics is as follows: ∆u=q-w , which means in a system is equal to added, minus done by the system. the units for these variables are in .
Answers: 1

Physics, 23.06.2019 01:30
Aplane's average speed between two cities is 600 km/h. if the trip takes 2.5 hours, how far does the plane fly?
Answers: 2
You know the right answer?
Acontainer of gas molecules is at a pressure of 2 atm and has amass density of 1.7 grams per liter....
Questions

History, 28.08.2019 14:00

Mathematics, 28.08.2019 14:00


History, 28.08.2019 14:00






Computers and Technology, 28.08.2019 14:00

History, 28.08.2019 14:00


History, 28.08.2019 14:00


Computers and Technology, 28.08.2019 14:00

History, 28.08.2019 14:00