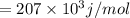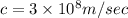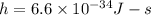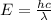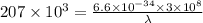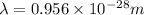Answers: 3


Another question on Physics

Physics, 22.06.2019 16:20
How does a circuit breaker protect a refrigerator? a. when the current is too high, a metal strip in the fuse melts and opens the circuit. b. when the resistance is too high , a re-settable which opens a circuit c. when the current is too high , a re-settable switch opens the circuit d. when the resistance is too high a metal strip in the fuse melts and opens the circuit
Answers: 2

Physics, 22.06.2019 19:30
Assume that two of the electrons at the negative terminal have attached themselves to a nearby neutral atom. there is now a negative ion with a charge -2e at this terminal. what are the electric potential and electric potential energy of the negative ion relative to the electron? the electric potential and the electric potential energy are both twice as much. the electric potential is twice as much and the electric potential energy is the same. the electric potential is the same and the electric potential energy is twice as much. the electric potential and the electric potential energy are both the same. the electric potential is the same and the electric potential energy is increased by the mass ratio of the oxygen ion to the electron. the electric potential is twice as much and the electric potential energy is increased by the mass ratio of the oxygen ion to the electron.
Answers: 3

Physics, 22.06.2019 20:00
Bahan yang digunakan mencegah terjadinya polarisasi pada batu baterai a.larutan h2so4b.mncl2 dan serbuk karbonc.pbso4d.nh4cl
Answers: 3

Physics, 23.06.2019 10:30
Up of elements with the same number of valence electrons. vertical column in the periodic table of elements such as alkali metals or halogens. a horizontal row of elements in the periodic table. this is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table. periodic trend these are the highly reactive elements located in group 1 of the periodic table. these elements have one electron in their outer energy level which makes them highly reactive with water and halogens. these are the reactive elements located in group 2 of the periodic table. these elements have two electrons in their outer energy level which makes them reactive with water and halogens. alkaline earth metals these are the group 3 or d-block elements. these dense metals with high boiling points can have different oxidation states and all are solid at room temperature with the exception of mercury. transition metals this is the highly reactive family of elements with 7 valence electrons. this is an element with full valence shell, very unreactive. this is a group of elements with few valence electrons that conducts heat and electricity. one of a class of elements having properties intermediate to metals and nonmetals. this is a type of element that has many valence electrons, not a conductor.
Answers: 1
You know the right answer?
The energy required to dislodge electrons from cesium metal via the photoelectric effect is 207 kj/m...
Questions

Mathematics, 03.11.2020 19:50

English, 03.11.2020 19:50

Mathematics, 03.11.2020 19:50

Chemistry, 03.11.2020 19:50

Mathematics, 03.11.2020 19:50



Health, 03.11.2020 19:50


Geography, 03.11.2020 19:50


Spanish, 03.11.2020 19:50

Computers and Technology, 03.11.2020 19:50


English, 03.11.2020 19:50

Mathematics, 03.11.2020 19:50

History, 03.11.2020 19:50

Chemistry, 03.11.2020 19:50


Social Studies, 03.11.2020 19:50