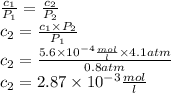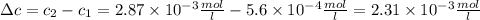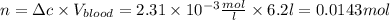The solubility of n2 in blood at 37°c and a partial pressure of 0.80 atm is 5.6 ✕ 10−4 mol·l−1. a deep-sea diver breathes compressed air with a partial pressure of n2 equal to 4.1 atm. assume that the total volume of blood in this diver's body is 6.2 l. calculate the amount of n2 gas released (in liters) when the diver returns to the surface of water, where the partial pressure of n2 is 0.80 atm. (2 sig fig)

Answers: 3


Another question on Physics

Physics, 21.06.2019 20:20
How much work would a child do while puling a 12-kg wagon a distance of 3m with a 22 n force directed 30 degrees with respect to the horizontal? (a) 82j (b) 52j (c) 109j (d) 95j
Answers: 2

Physics, 21.06.2019 21:30
Apendulum has a mass of 1.5 kg and starts at a height of 0.4 m. if it is released from rest, how fast is it going when it reaches the lowest point of its path? acceleration due to gravity is g = 9.8 m/s2. a. 2.8 m/s b. 0 m/s c. 5.9 m/s d. 4.3 m/s
Answers: 1

Physics, 22.06.2019 01:30
The transfer of heat through the movement of a gas or liquid a. convection b. conduction c. radiation
Answers: 2

Physics, 22.06.2019 05:30
Debbie places two shopping carts in a cart corral she pushes the first cart which then pushes a second cart what force is being exerted
Answers: 1
You know the right answer?
The solubility of n2 in blood at 37°c and a partial pressure of 0.80 atm is 5.6 ✕ 10−4 mol·l−1. a de...
Questions

History, 05.05.2020 20:59

Mathematics, 05.05.2020 20:59


Mathematics, 05.05.2020 20:59


Biology, 05.05.2020 20:59




Biology, 05.05.2020 21:00



Biology, 05.05.2020 21:00

History, 05.05.2020 21:00

Geography, 05.05.2020 21:00

Chemistry, 05.05.2020 21:00