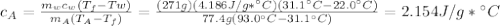Physics, 05.11.2019 00:31 haileyglowiak8183
A77.4-g sample of an alloy at 93.0°c is placed into 271 g of water at 22.0°c in an insulated coffee cup. assume that no heat is absorbed by the cup. if the final temperature of the system is 31.1°c, what is the specific heat capacity of the alloy in j/(g. k)? don't include units.

Answers: 2


Another question on Physics

Physics, 21.06.2019 17:30
Awheel rotates without friction about a stationary horizontal axis at the center of the wheel. a constant tangential force equal to 82.0 n is applied to the rim of the wheel. the wheel has radius 0.150 m . starting from rest, the wheel has an angular speed of 12.8 rev/s after 3.88 s. what is the moment of inertia of the wheel?
Answers: 3



Physics, 22.06.2019 18:50
An insulated thermos contains 148 g of water at 72.7 ˚c. you put in a 11.7 g ice cube at 0.00 ˚c to form a system of ice + original water. the specific heat of liquid water is 4190 j/kg•k; and the heat of fusion of water is 333 kj/kg. what is the net entropy change of the system from then until the system reaches the final (equilibrium) temperature?
Answers: 2
You know the right answer?
A77.4-g sample of an alloy at 93.0°c is placed into 271 g of water at 22.0°c in an insulated coffee...
Questions


Mathematics, 16.12.2019 21:31

Social Studies, 16.12.2019 21:31

Health, 16.12.2019 21:31

Mathematics, 16.12.2019 21:31

Social Studies, 16.12.2019 21:31

Mathematics, 16.12.2019 21:31


Chemistry, 16.12.2019 21:31

Mathematics, 16.12.2019 21:31

Mathematics, 16.12.2019 21:31

Chemistry, 16.12.2019 21:31

History, 16.12.2019 21:31

Mathematics, 16.12.2019 21:31

Mathematics, 16.12.2019 21:31

Mathematics, 16.12.2019 21:31




Mathematics, 16.12.2019 21:31

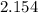
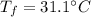 , mass of water
, mass of water 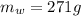 , specific heat capacity of water
, specific heat capacity of water 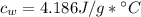 , initial temperature of water
, initial temperature of water 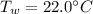 , mass of the alloy
, mass of the alloy 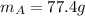 and initial temperature of the alloy
and initial temperature of the alloy 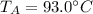 .
.