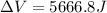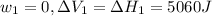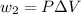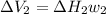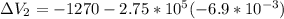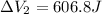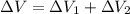Physics, 02.11.2019 07:31 chris018107
Find the total change in the internal energy of a gas that is subjected to the following two-step process. in the first step the gas is made to go through isochoric heating until 5460 j of heat is transferred into the gas and its pressure is 3.72 ✕ 105 pa. in the second step it is subjected to isobaric compression until its volume decreases by 7.50 ✕ 10−3 m3 and 1220 j of heat is transferred out of the gas. what is the total change in internal energy of this j?

Answers: 1


Another question on Physics

Physics, 21.06.2019 15:00
Aparticle moves according to a law of motion s = f(t), 0 ≤ t ≤ 12, where t is measured in seconds and s in feet. f(t) = cos(πt/6) (a) find the velocity at time t (in ft/s).
Answers: 3

Physics, 22.06.2019 04:10
Atotal charge of –6.50 µc is uniformly distributed within a sphere that has a radius of 0.150 m. what is the magnitude and direction of the electric field at 0.300 m from the surface of the sphere? a) 2.89 × 105 n/c, radially inward b) 6.49 × 105 n/c, radially outward c) 4.69 × 105 n/c, radially inward d) 9.38 × 105 n/c, radially outward e) 1.30 × 106 n/c, radially inward
Answers: 3

Physics, 22.06.2019 09:10
Which lists the organs in the correct order as food passes from the mouth to anus?
Answers: 1

Physics, 22.06.2019 10:00
In a heat engine if 1000 j of heat enters the system the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2000 j? 1. write the equation 2.list out your known variables 3.plug the numbers into the equations 4.solve 5.write your solution statement that includes initial energy and final
Answers: 3
You know the right answer?
Find the total change in the internal energy of a gas that is subjected to the following two-step pr...
Questions



Mathematics, 26.09.2019 22:40

Mathematics, 26.09.2019 22:40


Mathematics, 26.09.2019 22:40

Mathematics, 26.09.2019 22:40


Social Studies, 26.09.2019 22:40

Mathematics, 26.09.2019 22:40

Mathematics, 26.09.2019 22:40




Mathematics, 26.09.2019 22:40


Mathematics, 26.09.2019 22:40


Physics, 26.09.2019 22:40

History, 26.09.2019 22:40