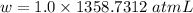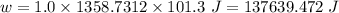Answers: 3


Another question on Physics

Physics, 22.06.2019 08:30
How is sound energy produced a. vibrating objects b. objects being heated c. temperature differences d. stationary objects
Answers: 2

Physics, 22.06.2019 09:30
The necleus of an atom is made up of what subatomic particles?
Answers: 1


Physics, 22.06.2019 14:40
Asolid cylinder and a cylindrical shell have the same mass, same radius, and turn on frictionless, horizontal axles. the cylindrical shell has light-weight spokes connecting the shell to the axle. a rope is wrapped around each cylinder and tied to blocks of equal masses that are held the same height above the ground. both blocks are released simultaneously. the ropes do not slip. which block hits the ground first? or is it a tie?
Answers: 3
You know the right answer?
Calculate the amount of pv work done on the surroundings when 1.00 kg of h2o absorbs energy from sun...
Questions




Mathematics, 19.02.2020 05:16

Mathematics, 19.02.2020 05:16



Mathematics, 19.02.2020 05:16

Mathematics, 19.02.2020 05:16

Mathematics, 19.02.2020 05:16

English, 19.02.2020 05:16




Mathematics, 19.02.2020 05:17





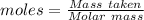
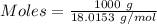
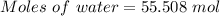
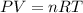
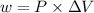
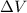 is the change in volume
is the change in volume