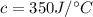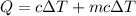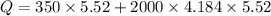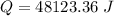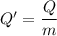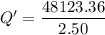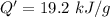Physics, 17.10.2019 20:20 awesomegrill
When 2.50 g of a certain hydrocarbon was completely combusted in a "bomb (constant-volume) calorimeter" with a heat capacity (excluding water) of 350 j/°c and which contained 2.00 liters of water (density = 1.00 g/ml and specific heat capacity = 4.184 j/°c•g), the resulting temperature change was measured to be 5.52°c. calculate the thermal energy (in kj) released per gram of hydrocarbon combusted. (1) 48.1 kj/g (2) 0.773 kj/g (3) 19.2 kj/g (4) 18.5 kj/g (5) 46.2 kj/g

Answers: 2


Another question on Physics

Physics, 22.06.2019 00:10
The summit of chimborazo, in ecuador, which is at a distance of about 6,384 km from the earth’s center. the bottom of the mariana trench, in the western pacific ocean, which is nearly 6,370 km from the center of the earth. on the surface of earth on the equator line.
Answers: 2

Physics, 22.06.2019 10:00
Explain the comparisons between nature and packingtown that appears on pages 81 and 82 answer
Answers: 2

Physics, 22.06.2019 13:00
Discuss how the hardness or softness of the landing surface is related to the time required to stop the egg
Answers: 1

Physics, 22.06.2019 16:00
From the perspective of an employee that effective channeling of work related information and concerns
Answers: 1
You know the right answer?
When 2.50 g of a certain hydrocarbon was completely combusted in a "bomb (constant-volume) calorimet...
Questions

Physics, 09.12.2020 19:00


Advanced Placement (AP), 09.12.2020 19:00



English, 09.12.2020 19:00

English, 09.12.2020 19:00

Physics, 09.12.2020 19:00






Advanced Placement (AP), 09.12.2020 19:00

Social Studies, 09.12.2020 19:00

Mathematics, 09.12.2020 19:00

Computers and Technology, 09.12.2020 19:00

English, 09.12.2020 19:00

Mathematics, 09.12.2020 19:00