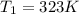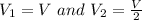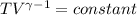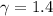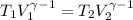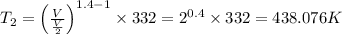Avertical cylinder is divided into two parts by a movable piston of mass m. the piston and cylinder system is well insulated (that is, no heat can flow in or out of the system) and the piston is initially held at rest. the top part of the cylinder is evacuated and the bottom part is filled with 1.00 mole of diatomic ideal gas at temperature 332 k. after the piston is released and the system comes to equilibrium, the volume occupied by gas is halved. find the final temperature of the gas.

Answers: 3


Another question on Physics

Physics, 21.06.2019 13:30
Abaseball is thrown a distance of 20 meters. what is its speed if it takes 0.5 seconds to cover the distance
Answers: 2

Physics, 21.06.2019 23:40
Aregular polygon has angkes of size 150° each.how many side has the polygon
Answers: 1

Physics, 22.06.2019 15:30
Ametal ring 4.20 cm in diameter is placed between the north and south poles of large magnets with the plane of its area perpendicular to the magnetic field. these magnets produce an initial uniform field of 1.12 t between them but are gradually pulled apart, causing this field to remain uniform but decrease steadily at 0.240 t/s . (a) what is the magnitude of the electric field induced in the ring? (b) in which direction (clockwise or counterclockwise) does the current flow as viewed by someone on the south pole of the magnet?
Answers: 2

Physics, 22.06.2019 22:00
Agirl throws a ball. if the ball's acceleration is 12 m/sec/sec and its mass is 0.5 kg, how much force did the girl apply to the ball?
Answers: 1
You know the right answer?
Avertical cylinder is divided into two parts by a movable piston of mass m. the piston and cylinder...
Questions









Mathematics, 08.02.2021 18:40


Chemistry, 08.02.2021 18:40





English, 08.02.2021 18:40

History, 08.02.2021 18:40



Arts, 08.02.2021 18:40