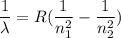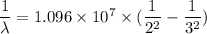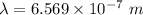The energy of the electron in a hydrogen atom can be calculated from the bohr formula: in this equation stands for the rydberg energy, and stands for the principal quantum number of the orbital that holds the electron. (you can find the value of the rydberg energy using the data button on the aleks toolbar.) calculate the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the electron from an orbital with to an orbital with . round your answer to significant digits.

Answers: 3


Another question on Physics

Physics, 21.06.2019 22:40
Consider two metallic rods mounted on insulated supports. one is neutral, the other positively charged. you bring the two rods close to each, but without contact, and briefly ground the the neutral rod by touching it with your hand. show answer correct answer what would be resulting charge (if any) on the initially neutral rod
Answers: 1

Physics, 22.06.2019 09:00
When a light bulb shines, it gives off light energy and energy. a. heat b. potential c. chemical d. electrical
Answers: 2

Physics, 22.06.2019 16:00
From the perspective of an employee that effective channeling of work related information and concerns
Answers: 1

Physics, 22.06.2019 20:30
What are some important factors to consider when choosing a warm-up before your workout?
Answers: 2
You know the right answer?
The energy of the electron in a hydrogen atom can be calculated from the bohr formula: in this equa...
Questions


Mathematics, 22.08.2019 03:20

Physics, 22.08.2019 03:20



Mathematics, 22.08.2019 03:20

Mathematics, 22.08.2019 03:20

Mathematics, 22.08.2019 03:20

Mathematics, 22.08.2019 03:20



Mathematics, 22.08.2019 03:20

Mathematics, 22.08.2019 03:20



History, 22.08.2019 03:20

Mathematics, 22.08.2019 03:20

Biology, 22.08.2019 03:20

Social Studies, 22.08.2019 03:20

Mathematics, 22.08.2019 03:20

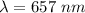
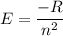 .............(1)
.............(1)