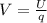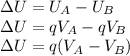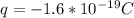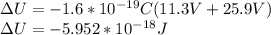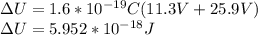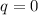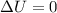Physics, 07.10.2019 18:30 penelopymorales
You cause a particle to move from point a, where the electric potential is 11.3 v, to point b, where the electric potential is −25.9 v. calculate the change that occurs in the particle's electrostatic potential energy, when the particle is an electron, a proton, a neutral hydrogen atom, and a singly ionized helium atom (i. e., lacking one electron from its neutral state).

Answers: 3


Another question on Physics

Physics, 22.06.2019 11:20
If a rock is thrown upward on the planet mars with a velocity of 12 m/s, its height (in meters) after t seconds is given by h = 12t − 1.86t2. (a) find the velocity of the rock after two seconds. m/s (b) find the velocity of the rock when t = a. 12−3.72a m/s (c) when will the rock hit the surface? (round your answer to one decimal place.) t = s (d) with what velocity will the rock hit the surface? m/s
Answers: 1

Physics, 22.06.2019 14:30
Two carts, one of mass 2m and one of mass m, approach each other with the same speed, v. when the carts collide, they hook together. assume that positive momentum is to the right. which graph best represents the momentum of both carts over time, before and after the collision?
Answers: 3

Physics, 22.06.2019 18:00
Air enters a gas turbine with two stages of compression and two stages of expansion at 100 kpa and 17°c. this system uses a regenerator as well as reheating and intercooling – the intercooler returns the air to the inlet temperature. the pressure ratio across each compressor is 4 ; 300 kj/kg of heat are added to the air in each combustion chamber; and the regenerator operates perfectly while increasing the temperature of the cold air by 20°c. determine the system’s thermal efficiency. assume isentropic operations for all compressor and the turbine stages and use constant specific heats at room temperature. (0.378)
Answers: 3

Physics, 23.06.2019 09:30
How many milliliters of water at 23 °c with a density of 1.00 g/ml must be mixed with 180 ml (about 6 oz) of coffee at 95 °c so that the resulting combination will have a temperature of 60 °c? assume that coffee and water have the same density and the same specific heat. how much will the temperature of a cup (180 g) of coffee at 95 °c be reduced when a 45 g silver spoon (specific heat 0.24 j/g °c) at 25 °c is placed in the coffee and the two are allowed to reach the same temperature? assume that the coffee has the same density and specific heat as water. a 45-g aluminum spoon (specific heat 0.88 j/g °c) at 24 °c is placed in 180 ml (180 g) of coffee at 85 °c and the temperature of the two become equal. (a) what is the final temperature when the two become equal? assume that coffee has the same specific heat as water. (b) the first time a student solved this problem she got an answer of 88 °c. explain why this is clearly an incorrect answer.
Answers: 1
You know the right answer?
You cause a particle to move from point a, where the electric potential is 11.3 v, to point b, where...
Questions





Mathematics, 24.06.2019 19:30




Mathematics, 24.06.2019 19:30




Biology, 24.06.2019 19:30


Mathematics, 24.06.2019 19:30




History, 24.06.2019 19:30

Biology, 24.06.2019 19:30