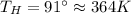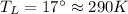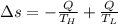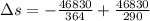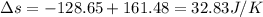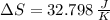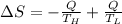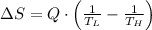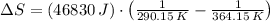Physics, 30.09.2019 23:30 noellelovebug1214
Suppose that there are two very large reservoirs of water, one at a temperature of 91.0 °c and one at a temperature of 17.0 °c. these reservoirs are brought into thermal contact long enough for 46830 j of heat to flow from the hot water to the cold water. assume that the reservoirs are large enough so that the temperatures do not change significantly. what is the total change in entropy resulting from this heat exchange between the hot water and the cold water?

Answers: 3


Another question on Physics

Physics, 22.06.2019 08:40
The system is released from rest with the cable taut, and the homogeneous cylinder does not slip on the rough incline. determine the angular acceleration of the cylinder and the minimum coeffi cient s of friction for which the cylinder will not slip.
Answers: 2

Physics, 22.06.2019 09:00
The material that keeps its new shape after it is stretched is called?
Answers: 1

Physics, 22.06.2019 12:50
Which changes would result in a decrease in the gravitational force btween two objects? check all that apply
Answers: 1

Physics, 22.06.2019 17:20
Which is not true of the intertropical convergence zone? a) it features heavy precipitation b) it's where the trade winds collidec) it's a high-pressure zone with sinking air d) it is also known as the doldrums
Answers: 2
You know the right answer?
Suppose that there are two very large reservoirs of water, one at a temperature of 91.0 °c and one a...
Questions

Social Studies, 30.09.2021 16:30

Mathematics, 30.09.2021 16:40

Mathematics, 30.09.2021 16:40





Mathematics, 30.09.2021 16:40



English, 30.09.2021 16:40

Mathematics, 30.09.2021 16:40

Engineering, 30.09.2021 16:40




Chemistry, 30.09.2021 16:40


Arts, 30.09.2021 16:40

Mathematics, 30.09.2021 16:40