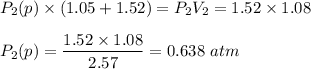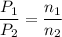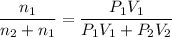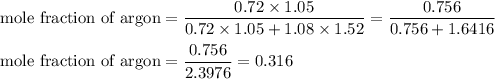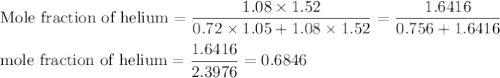A1.05-l bulb and a 1.52-l bulb are connected by a stopcock and filled, respectively, with argon at 0.72 atm and helium at 1.08 atm at the same temperature. calculate the total pressure, the partial pressures of each gas, and the mole fraction of each gas after the stopcock has been opened. assume ideal-gas behavior. (answer in 3 significant figures)

Answers: 3


Another question on Physics

Physics, 21.06.2019 18:10
Achild throws a ball with an initial speed of 8.00 m/s at an angle of 40.0° above the horizontal. the ball leaves her hand 1.00 m above the ground. how long is the ball in flight before it hits the ground?
Answers: 1

Physics, 21.06.2019 22:40
In physics the desire of an object to keep doing what it is doing is termed?
Answers: 1


Physics, 22.06.2019 04:20
Awave is produced in a rope. the wave has a speed of 33 m/s and a frequency of 22 hz.
Answers: 3
You know the right answer?
A1.05-l bulb and a 1.52-l bulb are connected by a stopcock and filled, respectively, with argon at 0...
Questions



Mathematics, 28.10.2019 07:31


World Languages, 28.10.2019 07:31





English, 28.10.2019 07:31

Mathematics, 28.10.2019 07:31

Mathematics, 28.10.2019 07:31



Mathematics, 28.10.2019 07:31


Mathematics, 28.10.2019 08:31



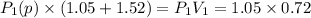
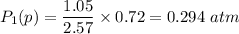
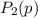 be the partial pressure of gas in bulb B then
be the partial pressure of gas in bulb B then